Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Pan-Amazônica de Saúde
versión impresa ISSN 2176-6215versión On-line ISSN 2176-6223
Rev Pan-Amaz Saude v.2 n.1 Ananindeua mar. 2011
http://dx.doi.org/10.5123/S2176-62232011000100004
ORIGINAL ARTICLE
Enteroparasitoses in a population of students from a public school in the Municipality of Mirassol, São Paulo State, Brazil
Marcus Vinicius Tereza BellotoI; Juares Elias Santos JuniorI; Elenir Alves MacedoI; Adão PonceII; Kátia Jaira GalisteuIII; Edna de CastroI; Luciana Ventura TauyrI; Andréa Regina Baptista RossitIV; Ricardo Luiz D. MachadoI
ICentro de Investigação de Microrganismos, Faculdade de Medicina de São José do Rio Preto, São José do Rio Preto-SP
IICurso de Enfermagem, UNIFAIMI- Mirassol-SP
IIIDepartamento de Enfermagem Geral, Faculdade de Medicina de São José do Rio Preto, São José do Rio Preto-SP
IVInstituto Biomédico, Universidade Federal Fluminense, Niterói-RJ
Endereço para correspondência
Correspondence
Dirección para correspondencia
Original Title: Enteroparasitoses numa população de escolares da rede pública de ensino do Município de Mirassol, São Paulo, Brasil. Translated by: American Journal Experts
ABSTRACT
This study observed the prevalence of intestinal parasites in 310 students (2 to 15 years old) enrolled in a public school in the Municipality of Mirassol, São Paulo State, Brazil. A stool sample was collected from each child and analyzed by the methods of Faust and Hoffmann, Pons and Janer, normally used for detection of protozoa and human helminths. A total of 30.3% of the children analyzed were parasitized, with at least one pathogenic intestinal parasite. Giardia Lamblia was the most common protozoan (15.16%), followed by Entamoeba histolytica (0.64%). The helminths found were Ascaris lumbricoides (3.55%), Strongiloides stercoralis and Taenia sp, which were diagnosed in 0.32% of the samples. There was a significant association between the occurrence of enteroparasitoses and the use of tap water. The comparison between the age groups, gender and the presence of parasites showed no statistical relevance. Although there was no association between gastrointestinal disorders and the occurrence of intestinal parasitic diseases, these agents may cause new infections because the children can act as carriers and therefore a source of contamination. This article suggests that a continuing education program focused on the prevention and treatment of parasitic infections is a key measure for their eradication.
Keywords: Enteroparasitoses; Giardia lamblia, Ascaris lumbricoides; Cross-Sectional Studies.
INTRODUCTION
Diseases that originate from enteric parasites are major public health problems worldwide and contribute to high rates of morbidity and mortality, especially in developing countries1,2. In these countries, it is estimated that approximately one-third of the population lives under environmental conditions that are favorable for the spread of parasitic infections3. Worldwide, infections by protozoa and helminths affect 3.5 billion people, and these organisms cause disease in approximately 450 million people4. In most cases, enteric parasites are transmitted orally by ingesting food or water that is contaminated with parasites. In Brazil, the wide diversity of socioeconomic characteristics, climate and geography are critical for the etiologic agent profile in diarrhea, thus modeling the frequency of these different enteric pathogens5,6.
Children comprise a high-risk group for infections by intestinal parasites7 because they can be exposed to parasites even when they are only a few months old8. Studies that searched for a positive correlation between the presence of enteric parasitic diseases and child gender9,10 and between those diseases and age range11,12 have presented inconclusive results. Furthermore, it has been found that good-quality water in daycare centers helps to prevent infections by enteric parasites, and this effect is more efficient when associated with sewer system of an equivalent quality13.
In Brazil, a wide variation in the frequency of enteric parasitism among the child population and in the agents involved, which can reach rates of nearly 80% in some regions, has been observed. Studies of enteric parasites in students from an outskirt of Maranhão State indicated that the Ascaris lumbricoides parasite was the most prevalent (40%)14, and this organism was also the most frequent in children from a rural region in the Municipality of Coari, Amazonas State, in Northern Brazil (67.5%)15. However, in the Municipality of Rio Verde, in Goiás State, a similar study found that the Giardia lamblia protozoan (59%) was the most prevalent12. In the Municipality of Criciúma in Santa Catarina State, Cryptosporidium (85.1%) was the most prevalent protozoan, and this organism was followed in prevalence by Entamoeba histolytica (56.4%) and G. lamblia (4.3%)6. Additionally, two other studies investigated the presence of E. histolytica11 and G. lamblia16 in children from a daycare center on the ou tskirts of Belém City, in Pará State, and detected the presence of these parasites in 21.8% and 26.9% of the samples, respectively.
In São Paulo State, this panorama remains unmodified because of the vulnerability of children to be infected by enteric parasites17. In children who were enrolled at a daycare center in the Municipality of Botucatu, in São Paulo State, giardiasis, enterobiasis and cryptosporidiosis, among other parasitic infections, were quite frequently observed18. Studies performed in northwestern São Paulo State19,20 have shown a high prevalence of enteric parasites in child populations, reaffirming that enteric parasites are a major public health problem. In the 1990s, an epidemiological survey conducted in the Municipality of Mirassol detected G. lamblia (61.1%), A. lumbricoides (2.8%) and Ancylostoma hookworms (3.2%) in children13,21.
The objective of this study was to evaluate the prevalence of enteric parasites in students at public schools in the Municipality of Mirassol and to investigate the possible epidemiological associations with their socioeconomic status.
MATERIAL AND METHODS
From September 2009 to March 2010, fecal samples from students who were enrolled in a public school in the Municipality of Mirassol, in São Paulo State, were analyzed. The school is located on the city outskirts and originated from an urbanized slum (a Brazilian favela). It serves children who are in the 1st through 4th grades of primary school from 19 different communities.
After a detailed explanation of the project was given and consent signatures from the guardians of the children were obtained, a single feces sample was collected from each child and placed in 10% formalin, and a socio-epidemiological data questionnaire was filled out. The samples were sent to a laboratory at the Center for Microorganism Research at the São José do Rio Preto College of Medicine (Faculdade de medicina de São José do Rio Preto - FAMERP), where parasitological examinations were conducted. Methods used for enteric parasite detection included techniques by Faust, which are based on centrifugal flotation, and that by Hoffmann, Pons and Janer, which is based on spontaneous sedimentation; both types of techniques are usually conducted to detect human protozoa and helminths. Laboratory analyses were performed at the Center for Microorganism Research, where we searched for a correlation between the parasitological results and the socioeconomic status of each child. The following conditions were included in the study: the type of food consumed, water consumption, gender, age range, family income and the educational level of the parents or guardians. In addition, we investigated the association between gastroenteric disorders and parasites that were detected in diarrheal and non-diarrheal feces.
To determine the statistical significance between the studied groups, chi-square (χ2) and Fisher's exact tests were conducted using the EPIINFO statistical software, version 6.0. Differences between the groups were considered statistically significant if p < 0.05. The project was approved by the Ethics in Research Committee of FAMERP (CEP/FAMERP in 5159/2009).
RESULTS
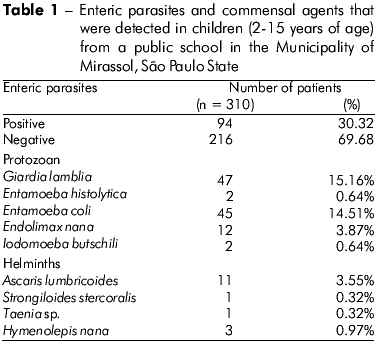
Fecal samples from 310 children were analyzed. As summarized in Table 1, 30.32% (94/310) were infected with at least one pathogenic, enteric parasite. The G. lamblia protozoan was found most frequently (15.16%), and the second most frequent pathogen that was identified was E. histolytica (0.64%). The following helminths were detected: A. lumbricoides (3.55%), S. stercoralis and Taenia sp. (0.32%).
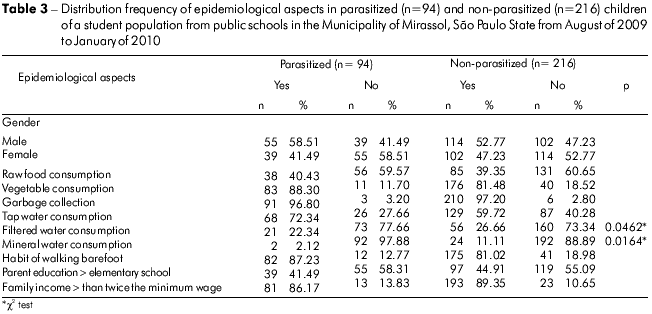
The participants were classified according to age range: 2 to 4 years (n = 39), 5 to 7 years (n = 127), 8 to 10 years (n = 114) and 11 to 15 years (n = 30). The 8- to 10-year-old group exhibited the highest number of positive samples (47.37%), followed by the 2- to 4-year-old (38.46%), 5- to 7-year-old (36.22%) and 11- to 15-year-old (30.0%) groups. There was no significant difference between the age ranges and presence of parasites (Table 2). However, a significant association was observed between tap water consumption and the presence of enteric parasites (p = 0.0462). There was no significant relationship between child gender and the occurrence of enteric parasites (Table 3).
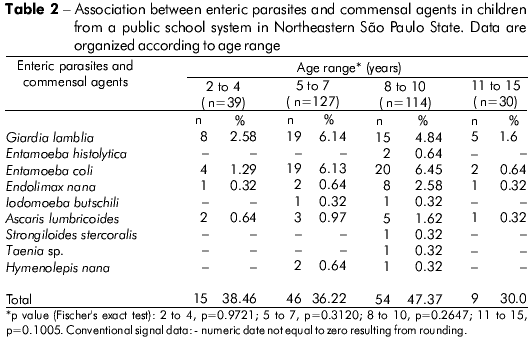
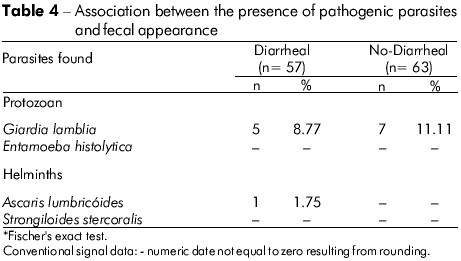
A subset of samples (n = 120) was investigated to establish the relationship between fecal appearance and parasitism, but no statistical significance was found (Fisher's exact test, p = 0.7226) (Table 4).
DISCUSSION
Infections caused by enteric pathogens are a basic, public health problem in tropical regions22 and have been reported to be responsible for child diarrhea23. In Latin America, the high diversity of geographical and socioeconomic characteristics is described as a factor that influences the etiology of infectious diarrhea and modulates the various enteric pathogens that cause this disorder24. Results from this study demonstrate a 30.3% parasitism rate in the studied population, and the organisms that were found most frequently were G. lamblia (15.16%) and A. lumbricoides (3.55%). In other studies on Brazilian children, the frequency of enteric and commensal parasites varied from 24.6%25 to 92%26. Interestingly, in an investigation that was performed a decade ago with students from this municipality, 63.9% of the population was parasitized, and the same parasites were the most prevalent21. The lower frequency of parasitic diseases that is currently observed may be related to the fact that only one fecal sample from each child was examined. However, the percentage of samples that were positive for enteric and/or commensal parasites found in this study reflects the individuals' exposure to contaminated soil and their poor hygiene habits.
It is known that the frequency of giardiasis is higher in developing countries than in developed countries. Some authors claim that this protozoiasis, unlike the helminthiases, is present at a higher frequency in children from higher income families due to higher consumption of garden vegetables27,28. Furthermore, a reduced giardiasis rate normally occurs with increasing age range because successive contact with the parasite increases the host's immunity and because hygiene becomes more effective as the child grows29,30. Another important factor in the spread of giardiasis is that this parasite is often found in collective environments, and its transmission by direct, person-to-person contact increases the chances of contamination21. These results depict rates that are similar to those described among the general Brazilian population16. However, we cannot rule out the possibility that the detected giardiasis rates may be related to the parasite's biological characteristics, such as its intermittent elimination. As mentioned earlier, the fact that only one sample was collected per child may have contributed to this casuistry in children.
E. histolytica is the only species of amoeba that is considered invasive, and it is highly prevalent in tropical regions, especially in communities with inadequate sanitary conditions31. In several countries, commensal amoebae infect people, but most of these individuals are asymptomatic. These results exhibit the low casuistry of this parasite, indicating that it may not be endemic to the region. However, detection of commensal amoebae, such as Entamoeba coli, Endolimax nana and lodamoeba butschlii, indicates that those children had ingested water or food that had been contaminated with fecal waste and were at risk for infection by E. histolytica. These data reinforce the importance of diagnosis and description of these commensals to implement preventative measures for infection due to oral-fecal contamination by pathogenic amoebae.
Several studies have related infections by A. lumbricoides with reduced growth and storage proteins in children and adolescents. Causes of this disorder include reduced enteric absorption and lumen obstruction, which leads to anorexia and blocking of surface absorption32. Strategies to control the occurrence of this geo-helminth have indicated that, in addition to age, the number of people living within the same residence is an important factor in determining the distribution of the parasite among families33. A. lumbricoides was the most diagnosed helminth in this study, which was different from what had been found in other regions of Brazil24. However, a previous study found a low frequency of this parasite in students from the Mirassol21 region, which leads us to believe that this parasite is not a problem in this community.
Only one case of infection with S. stercoralis, in a child who did not normally use footwear, was diagnosed in this study. In fact, several authors have described low levels of infections caused by this helminth in children10,24,25. However, because most of the population who was evaluated in this study exhibited a habit of walking barefoot, more attention should be paid to this type of parasitism so that this type of infection does not become a future problem.
Taeniasis is an important public health problem in urban and rural areas34, and cysticercosis is a parasitic disease that is also caused by human taeniid cestodes. The transmission of these organisms is facilitated by the presence and availability of eggs in water and food35. In this study, only one case of tapeworm infection was found, which corroborated with the literature showing low frequencies of this parasite in children36. Notably, this case was diagnosed in a student who had a backyard garden. The direct association between human and swine infection, especially in places where these mammals coexist, favors enteric parasite transmission37. Therefore, careful delineation of land parcels and gardens that are subject to animal traffic, especially of pigs, can prevent the endemicity of the taeniasis/cysticercosis complex in this region.
Brazilian literature has indicated that consumption of raw food, such as fruit and vegetables, that is contaminated with human fecal matter contributes to the transmission of several enteric parasites13,38. A dietary habit that consists of raw garden vegetables exposes a large portion of the population to transmissible forms of parasites39. However, the results of this study found no statistical significance of this variable. In contrast, there was a significant association between tap water consumption and infection by enteric parasites. It is known that the enteric parasites detected in this study are mostly waterborne, and a previous study showed that children who consumed unfiltered water were 15.9 times more likely to acquire parasitic diseases13. However, there is an official water treatment system in the tested municipality. Therefore, how potable water for residences i s being stored should be investigated to understand why it is a risk factor for infection of children.
It is known that enteric parasites can cause significant health problems, such as malnutrition, anemia, enteric obstruction and diarrhea, especially in children40,41. Diarrhea may or may not be infectious42. However, the fact that no significant result was found between the presence of enteric parasites and this clinical condition suggests that there are other reasons for the occurrence of this enteric condition in children. Furthermore, studies on the etiologic agents that are associated with diarrhea have shown that the relative importance of different enteric pathogens varies greatly, depending on the season, residence (urban or rural) areas, socioeconomic status, geographic location and especially host age5,6,43. In addition, cases of diarrhea may be associated with other nosologies or other enteric pathogens, such as viruses and bacteria, or other uninvestigated protozoa, such as Isospora belli and Cryptosporidium44. In contrast, it should be noted that asymptomatic infections might also be due to mechanisms of immune tolerance or intraspecific variation that may affect parasite virulence42.
CONCLUSION
Finally, we must consider that due to constant socio-demographic changes that are observed worldwide, different characteristics of circulating diseases, as well as new pathogenic microorganisms in humans45, emerge. Although there have been advances in the treatment and diagnosis of these diseases in recent years, enteroparasitic diseases remain a significant public health problem, especially in developing countries. In addition, control actions are still restricted by the basic sanitation infrastructure and the lack of educational projects that could inform the population about these diseases. Despite the identification of enteric parasites that are not associated with gastroenteric disorders in this study, the presence of these agents may lead to new cases because these children can act as carriers and, therefore, sources of contamination. The results from this study lead us to suggest that a continued awareness program involving prevention and treatment of parasitic infections is a key measure for the eradication of these diseases.
ACKNOWLEDGEMENTS
We would like to thank the following professionals at the Center for Microorganism Research, FAMERP, Gustavo Capatti, Luciane Storti, Luciana Moran, Valéria Fraga and Amanda Oliveira, for assistance in sample collection and technical support. We would also like to thank the employees at the Municipal School of Mirassol, who provided consent for this project to be performed.
REFERENCES
1 Rocha A, Mendes RA, Barbosa CS. Strongyloides spp e outros parasitos encontrados em alfaces (lactuca sativa) comercializados na cidade do Recife, PE. Rev Patol Trop. 2008 maio-jun;37(2):151-60. [Link]
2 Dagci H, Kurt O, Demirel M, Ostan I, Azizi NR, Mandiracioglu A, et al. The prevalence of intestinal parasites in the province of Izmir, Turkey. Parasitol Res. 2008 Sep;103(4):839-45. Doi:10.1007/s00436-008-1065-6. [Link]
3 Soldan OCP, Vásquez FV, Varas AG, Cordón GP, Soto JR V, Sánchez-Moreno M. Intestinal parasitism in Peruvian children and molecular characterization of Cryptosporidium species. Parasitol Res. 2006 May;98(6):576-81. Doi:10.1007/s00436-005-0114-7 [Link]
4 Organização Mundial de Saúde. Division of Control of Tropical Diseases. Intestinal Parasites Control: geographical distribution 2006. Disponível em: http://www.who.int/ctd/html/intestburtre.html.
5 Cimerman S, Cimerman B, Lewi DS. Avaliação da relação entre parasitoses intestinais e fatores de risco para o HIV em pacientes com AIDS. Rev Soc Bras Med Trop. 1999 mar-abr;32(2):181-5. Doi:http://dx.doi.org/10.1590/S0037-86821999000200010 [Link]
6 Schnack FJ, Fontana LM, Barbosa PR, Silva LSM, Baillargeon CMM, Barichello T, et al. Enteropatógenos associados com diarréia infantil ( < 5 anos de idade) em amostra da população da área metropolitana de Criciúma, Santa Catarina, Brasil. Cad Saude Publica. 2003 jul-ago;19(4):1205-8. [Link]
7 Gurgel RQ, Cardoso GS, Silva AM, Santos LN, Oliveira RCV. Creche: ambiente expositor ou protetor nas infestações por parasitos intestinais em Aracaju, SE. Rev Soc Bras Med Trop. 2005 mai-jun;38(3):267-9. Doi:http://dx.doi.org/10.1590/S0037-86822005000300013 [Link]
8 Coulter JBS. Global importance on parasitic disease. Current Paediatrics. 2002 Dec;12(7):523-33. Doi:10.1054/cupe.2002.0352 [Link]
9 Toledo MJO, Paludetto AW, Moura FT, Nascimento ES, Chaves M, Araújo SM, et al. Evaluation of enteroparasite control activities in a Kaingáng community of Southern Brazil. Rev Saude Publica. 2009 Dec;43(6):981-90. [Link]
10 Machado ER, Santos DS, Costa-Cruz JM. Enteroparasites and commensals among children in four peripheral districts of Uberlândia, State of Minas Gerais. Rev Bras Med Trop. 2008 Nov-Dec;41(6):581-5. Doi:http://dx.doi.org/10.1590/S0037-86822008000600007 [Link]
11 Povoa MM, Arruda JEG, Silva MCM, Bichara CNC, Esteves P, Gabbay YB. Diagnóstico de amebíase intestinal utilizando métodos coproscópicos e imunológicos em amostra da área metropolitana de Belém, Pará, Brasil. Cad Saude Publica. 2000 jul-set;16(3):843-6. [Link]
12 Zaiden MF. Enteroparasiotses em crianças de 0 a 6 anos de creches municipais de Rio Verde-GO e sua interface com o meio ambiente. [dissertação]. São Paulo (SP): Universidade de Franca; 2006.
13 Komagome SH, Romagnoli MPM, Previdelli ITS, Falavigna DLM, Dias MLGG, Gomes ML. Fatores de risco para infecção parasitária intestinal em crianças e funcionários de creche. Cienc Cuid Saude. 2007;6 Suppl 2:S442-7. [Link]
14 Silva-Souza N, Ferreira MS, Cavalcante AN, Costa DS, Silva SEFC, Moraes EC, et al. Ocorrência de enteroparasitoses em escolares da periferia da Universidade Estadual do Maranhão. Rev Pesq Foco. 2008; 16(1):7-14. Nota de Pesquisa. [Link]
15 Silva EF, Silva EB, Almeida KS, Sousa JJN, Freitas Filho LC. Enteroparasitoses em crianças de áreas rurais do Munícipio de Coari, Amazonas, Brasil. Rev Patol Trop. 2009 jan-mar;38(1):35-43.
16 Machado RLD, Figueredo MC, Frade AF, Kudó ME, Silva Filho MG, Povoa MM. Comparação de quatro métodos laboratoriais para diagnóstico da Giardia lamblia em fezes de crianças residentes em Belém, Pará. Rev Soc Bras Med Trop. 2001 jan-fev;34(1):91-3. [Link]
17 Mascarini LM, Donalísio MR. Giardiasis and cryptosporidiosis in children institutionalized at daycare centers in the state of São Paulo. Rev Soc Bras Med Trop. 2006 Nov-Dec;39(6):577-9. Doi:http://dx.doi.org/10.1590/S0037-86822006000600015 [Link]
18 Carvalho TB, Carvalho LR, Mascarini LM. Occurrence of enteroparasites in day care centers in Botucatu (São Paulo State, Brazil) with emphasis on Cryptosporidium sp., Giardia duodenalis and Enterobius vermicularis. Rev Inst Med Trop Sao Paulo. 2006 Sep-Oct;48(5):269-73. [Link]
19 Malta RCG. Estudo epidemiológico dos parasitas intestinais em crianças no Município de Votuporanga [dissertação]. Campinas (SP): Universidade Federal de Campinas, Instituto de Biologia; 2006. [Link]
20 Mascarini LL, Donaliso-Cordeiro MR. Helmintíases em crianças institucionalizadas em creches no Município de Botucatu/SP, Brasil. Rev Patol Trop. 2007 maio-ago;36(2):149-58. [Link]
21 Machado RC, Marcari EL, Cristante SFV, Carareto CMA. Giardíase e helmintíases em crianças de creches e escolas de 1º e 2º graus (públicas e privadas) da cidade de Mirassol (SP, Brasil). Rev Soc Bras Med Trop. 1999 nov-dez;32(6):697-704. [Link]
22 Kumar A, Agarwal S, Heyman JA, Matson S, Heidtman M, Piccirillo S, et al. Subcellular localization of the yeast proteome. Genes Dev. 2002 Mar;16(6):707-19. Doi:10.1101/gad.970902 [Link]
23 Aslani MM, Alikhani MY, Zavari A, Yousefi R, Zamani AR. Characterization of enteroaggregative Escherichia coli (EAEC) clinical isolates and their antibiotic resistance pattern. Int J Infect Dis. 2011 Feb;15(2):e136-9. [Link]
24 Miné JC, Rosa JA. Frequency of Blastocystis hominis and other intestinal parasites in stool samples examined at the Parasitology Laboratory of the School of Pharmaceutical Sciences at the São Paulo State University, Araraquara. Rev Soc Bras Med Trop. 2008 Nov-Dec;41(6):565-9.[Link]
25 Menezes AL, Lima VMP, Freitas MTS, Rocha MO, Silva EF, Dolabella SS. Prevalence of Intestinal Parasites in Children from Public Daycare Center in the City of Belo Horizonte, Minas Gerais, Brazil. Rev Inst Med Trop S Paulo. 2008 Jan-Feb;50(1):57-9. [Link]
26 Florêncio MLQ. Estudo de alguns aspectos epidemiológicos das enteroparasitoses em famílias da cidade de Pradópolis, São Paulo. J pediatr (Rio J.). 1986 jun;60:291-6. [Link]
27 Marzochi MCA, Carvalheiro JR. Estudos dos fatores envolvidos na disseminação dos enteroparasitas. Rev Inst Med Trop São Paulo. 1978 jan-fev;20(1):31-5.
28 Santos RCV, Hoerlle JL, Aquino ARC, Carli GA. Prevalência de enteroparasitoses em pacientes ambulatoriais do Hospital Divina Providência de Porto Alegre, RS. Rev bras anal clin. 2004;36(4):241-3. [Link]
29 Tashima NT, Simões MJS. Parasitas intestinais: prevalência e correlação com a idade e com os sintomas apresentados de uma população infantil de Presidente Prudente – SP. Rev Bras Anal Clin. 2005;37(1):35-9. [Link]
30 Mukherjee AK, Chowdhury P, Bhattacharya MK, Ghosh M, Rajendran K, Ganguly S. Hospital-based surveillance of enteric parasites in Kolkata. BMC Res Notes. 2009 Jun;2:110. Doi:10.1186/1756-0500-2-110 [Link]
31 Stephenson LS, Latham MC, Ottesen EA. Malnutrition and parasitc helminth infections. Parasitology. 2000;121 Supl:S23-38. [Link]
32 Haswell-Elkins M, Elkins D, Anderson RM. The influence of individual, social group and household factors on the distribution of Ascaris lumbricoides within a community and implications for control strategies. Parasitology. 1989 Feb;98 ( Pt 1):125-34. [Link]
33 Sarti E. La teniosis y cisticercosis por Taenia solium. Salud pública Méx. 1997 mayo-jun;39(3):225-31. [Link]
34 Saldiva SRM, Carvalho HB, Castilho VP, Struchiner CJ, Massad E. Malnutrition and susceptibility to enteroparasites: reinfection rates after mass chemotherapy. Paediatr Perinat Epidemiol. 2002 Apr;16(2):166-71. [Link]
35 Marques SMT, Bandeira C, Quadros RM. Prevalência de enteroparasitoses em Concórdia, Santa Catarina, Brasil. Parasitol latinoam. 2005;60:78-81.
36 Praet N, Kanobana K, Kabwe C, Maketa V, Lukanu P, Lutumba P, et al. Taenia solium cysticercosis in the Democratic Republic of Congo: how does pork trade affect the transmission of the parasite? PLoS Negl Trop Dis. 2010 Sep;4(9):pii:e817. [Link]
37 Benetton MLFN, Gonçalves AV, Meneghini MEF, Silva EF, Carneiro M. Risk factors for infection by the Entamoeba histolytica/E. dispar complex: an epidemiological study conducted in outpatient clinics in the city of Manaus, Amazon Region, Brazil. Trans R Soc Trop Med Hyg. 2005 Jul;99(7):532-40. [Link]
38 Cantos GA, Soares B, Maliska C, Glick D. Estruturas parasitárias encontradas em hortaliças comercializadas em Florianópolis, Santa Catarina. Rev News Lab. 2004;66:154-63. [Link]
39 Brooker S, Alexander N, Geiger S, Moyeed RA, Stander J, Fleming F, et al. Contrasting patterns in the small-scale heterogeneity of human helminth infections in urban and rural environments in Brazil. Int J Parasitol. 2006 Sep;36(10-11):1143-51. [Link]
40 Saldiva SRM, Carvalho HB, Castilho VP, Struchiner CJ, Massad E. Malnutityion and susceptibility to enteroparasites: reinfection rates after mass chemotherapy. Paediatr Perinat Epidemiol. 2002 Apr;16(2):166-71. DOI:10.1046/j.1365-3016.2002.00402.x [Link]
41 Rossit AR, Almeida MT, Nogueira CA, Oliveira JG, Barbosa DM, Moscardini AC, et al. Bacterial, yeast, parasitic, and viral enteropathogens in HIV-infected children from São Paulo State, Southeastern Brazil. Diagn Microbiol Infect Dis. 2007 Jan;57(1):59-66. [Link]
42 Cimerman S, Cimerman B, Lewi DS. Prevalence of intestinal parasitic infections in patients with acquired immunodeficiency syndrome in Brazil. Int J Infect Dis.1999 Summer;3(4):203-6. [Link]
43 Bresee JS, Hummelman E, Nelson EA, Glass RI. Rotavirus in Asia: the value of surveillance for informing decisions about the introduction of new vaccines. J Infect Dis. 2005 Sep;192 Suppl 1:S1-5. Doi:10.1086/431515. [Link]
44 Weiss A, Bates TC, Luciano M. Happiness is a personal(ity) thing: the genetics of personality and well-Being in a representative sample. Psychol Sci. 2008 Mar;19(3):205-10. [Link]
 Correspondence / Correspondência / Correspondencia:
Correspondence / Correspondência / Correspondencia:
Marcus Vinicius Tereza Belloto
Centro de Investigação de Microrganismos,
Departamento de Doenças Infecciosas e Parasitárias,
Faculdade de Medicina-São José do Rio Preto.
Av. Brigadeiro Faria Lima, 5416 - Vila São Pedro - 15090-000
São José do Rio Preto – SP
Telefone: 017-32015736
e-mail:marcusbelloto@hotmail.com
Recebido em / Received / Recibido en: 10/12/2010
Aceito em / Accepted / Aceito en: 4/3/2011










 texto en
texto en