Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Pan-Amazônica de Saúde
versión impresa ISSN 2176-6215versión On-line ISSN 2176-6223
Rev Pan-Amaz Saude v.2 n.1 Ananindeua mar. 2011
http://dx.doi.org/10.5123/S2176-62232011000100009
ORIGINAL ARTICLE
Laboratory analysis of the flight of Rhodnius brethesi Matta, 1919, potential wild vector of Trypanosoma cruzi in the Brazilian Amazon. (Hemiptera:Reduviidae:Triatominae)
Dayse da Silva RochaI; Claudia SolanoII; José JurbergI; Vanda CunhaI; Cleber GalvãoI
ILaboratório Nacional e Internacional de Referência em Taxonomia de Triatomíneos, Instituto Oswaldo Cruz/MS, Rio de Janeiro, Rio de Janeiro, Brasil
IIDepartamento de Ciências Biológicas, Faculdade de Ciências Farmacêuticas, Universidade Estadual Paulista, Araraquara, São Paulo, Brasil
Endereço para correspondência
Correspondence
Dirección para correspondencia
ABSTRACT
The flight ability of Rhodnius brethesi was evaluated through laboratory observations, and it was correlated with the quantity of blood ingested, gender and fasting period. A total of 65 insects were analyzed: 27 males and 38 females; their mean survival period was 17.8 and 22.3 days, respectively. Most of the insects started flying after 13 days.
Keywords: Rhodnius; Chagas Disease; Triatominae; Insect Vectors; Behavior; Animal; Flight, Animal.
INTRODUCTION
Triatominae bugs (Hemiptera: Reduviidae) are the vectors of Chagas disease. A total of 141 species, grouped into five tribes and 18 genera, are currently known1,2,3, including the first fossil species, which was recently described4.
Rhodnius brethesi, known locally as "piolho da piaçaba" (piassaba lice), is a sylvatic species found in the native palm tree piassaba Leopoldina piassaba, in Amazonas State, Brazil. Transmission of Trypanosoma cruzi to families working on the extraction of piassaba fibers has been recorded in the Municipality of Barcelos, north of the Negro River, in the natural ecotope of R. brethesi5,6,7,8.
Infection of humans, reservoirs and vectors with T. cruzi and autochthonous cases of Chagas disease have been reported in most countries in the Amazon Basin. Interest in Chagas disease has increased over the past two decades in this area. The Amazon Region is characterized by the absence of vector domiciliation and dispersal of native species, mostly within the genus Rhodnius. This situation may change due to continuous deforestation and the immigration of human populations from other regions.
Passive dispersal of triatomines depends on the habits and behavior of their hosts. Several authors have reported the involuntary transport of triatomines in clothing, utensils and vehicles9. The feathers of migratory birds also act as excellent vehicles for the dispersal of eggs and even nymphs10,11,12,13. Active dispersal may occur by walking, for nymphs and adults, but mostly occurs by adult flight.
Abrahan et al14 have recently analyzed active dispersal (by flying and walking) and the role of chickens as passive carriers of Triatoma infestans in Argentina. For the first time, the authors reported the recapture of females that dispersed by walking and suggested that it is unlikely that chickens passively transport the insects. From an epidemiological perspective, dispersal is of great importance to the spread of Chagas disease, especially in treated areas that may experience recolonization.
Flight initiation in triatomines may be induced by environmental factors, such as temperature or nutritional status. Field and laboratory experiments performed by Lehane et al15 have shown that flight initiation in triatomines is primarily associated with nutritional status and increased environmental temperature. However, Soares and Santoro16 have observed that some species do not initiate flight under any conditions. During the imaginal ecdysis of triatomines, flight ability appears to be a result of morphological changes and the adaptation of muscles directly involved in this activity.
The flight activity of R. brethesi was evaluated in the laboratory and correlated with sex, the amount of blood ingested and fasting period duration.
MATERIALS AND METHODS
The experiment was performed using stage-5 specimens of R. brethesi from a colony reared from insects captured in piassaba trees by the Araca River, a tributary of the Negro River (Amazonas State, Brazil), in 2004 and acclimated to the laboratory. The specimens selected for the rearing of adults were fully engorged after a 4-hour feeding period. After the imaginal ecdysis, the insects (27 males and 38 females) were weighed before and after feeding to determine the amount of blood ingested and were individually marked with gouache on the pronotum and scutellum using a color code based on the one proposed by MacCord et al17. All insects were then placed inside a device designed by Galvao et al18 for the observation of triatomine flight. The insects were observed on a daily basis until they died, and the flights of flying specimens were recorded. The entire experiment was conducted at 26 ± 5o C and 70 ± 5% relative humidity (RH). Wingless insects were used as a control group. The insects were fed on pigeons (Columba livia) that were properly immobilized and anesthetized in accordance with protocol L-081-08, which was approved by the Ethics Committee on the Use of Animals (CEUA-Fiocruz). Statistical analyses were performed using unpaired t tests with equal SDs and the GraphPad Instat 3.06 software.
RESULTS
The mean lifespan of adult R. brethesi individuals was 17.8 days for males and 22.3 days for females, and the difference was statistically significant according to the unpaired t test performed (Table 1).
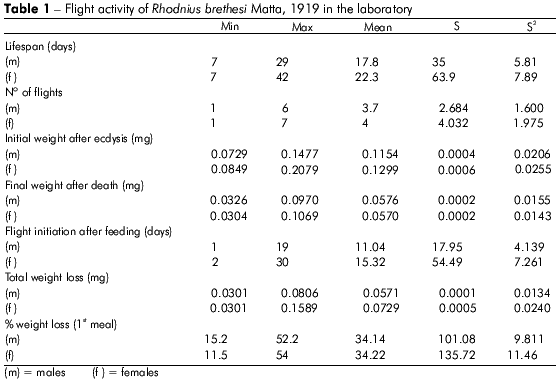
There was no significant difference in the number of flights between male and female individuals, despite the significantly greater initial weight of the females.
Females ingested significantly more blood than male individuals and also exhibited higher weight loss than male insects during their significantly longer lifespan (P = 0.0035).
The insects required an average of 13 days after the imaginal ecdysis to start flying. Flight initiation took significantly longer in female individuals (15 days) than males.
There were significant differences in flight time, initial weight and time to first flight between male and female individuals. Flight time was 3.58% longer in females than in males; initial weight was 11.53% higher in females than in males; and time to first flight was 4.7% higher in females than in males. Females that flew more frequently were initially heavier and initiated flight earlier than males.
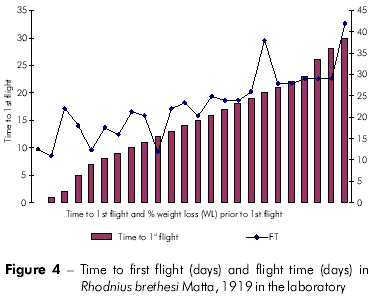
Plots showing the correlation between the number of flights and total weight loss; flight time and initial weight; % weight loss to first flight and time to first flight; and flight time and time to first flight are displayed in figures 1, 2, 3, and 4, respectively, and the Pearson coefficients and P values for the comparative analyses are shown in table 2.
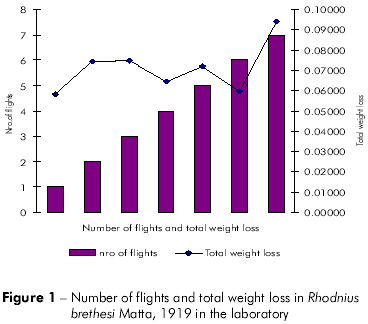
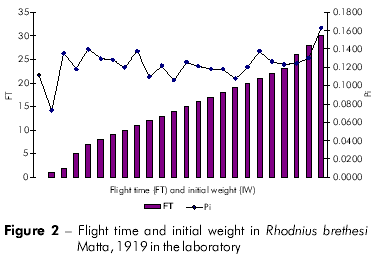
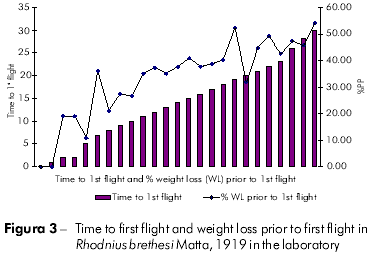

The Pearson coefficient (r) ranges from +1 to -1, and the closer the results are to these values, the stronger the association between the variables tends to be.
Individuals who took longer to initiate the first flight also flew more frequently. Moreover, these individuals also exhibited a higher percentage of weight loss. The time to first flight X lifespan and time to first flight X percentage weight loss to first flight variables were strongly associated.
Individuals who flew longer exhibited higher initial weight than other individuals, but the difference was not significant (P = 0.4133). Furthermore, total weight loss was higher in individuals who flew more frequently, but this difference was not statistically significant (P = 0.2280).
DISCUSSION
The small supply of hosts in its natural ecotope may have caused adaptations to the feeding behavior of R. brethesi, which has previously been observed feeding on piassaba collectors during the day. Rocha et al19 studied the life cycle of the species in the laboratory and found that the insects were extremely efficient in using blood meals, with only one meal needed for ecdysis to the fifth nymph stage. This swift and efficient feeding mode would enable the insects to feed during the day using humans as a host. In 1922, Matta20 reported on the aggressiveness of R. brethesi for the first time; these insects sucked on the entire body of piassaba collectors, causing secondary inflammation in their skin.
The mean lifespans in this study (17.8 days for males and 22.3 days for females) were much lower than the lifespans observed by Mascarenhas21,22, (51.5 days for males and 57.5 days for females) under similar conditions (26o C, 75% RH). Silva and Silva23 also observed a longer lifespan (45.2 days for males and 38.9 days for females), although at a higher temperature (30o C, 70 ± 5% HR). These results suggest that the short lifespan recorded in this study was caused by the nutritional restriction the individuals experienced and fatigue due to flight attempts.
When flight initiation and feeding were examined, some individuals were observed initiating flight on the same day as feeding. This result was unexpected, as several authors have associated flight initiation with low nutritional status and high temperatures15,24. Moreover, the time to first flight X lifespan and time to first flight x percentage weight loss to first flight were strongly associated.
Of the 65 individuals studied, 12 (18%) did not fly under any conditions, thus indicating that intraspecific characteristics may also influence flight behavior in triatomines, as reported by Soares and Santoro16. Several studies have shown that each species has a specific profile, and further characterization may help to identify species with greater active dispersal ability. Several authors have also associated the absence or reduction of flight muscles with the lack of flight initiation24,25.
A greater number of flights by females was also observed in Triatoma infestans by Gurevitz et al25, who found that the absence of flight muscles was 2.4 times more frequent in males than in females.
Rocha et al19 observed an average of seven blood meals during the adult stage of R. brethesi, which corresponds to approximately 50% of all flight attempts in the species. Weight loss measurements indicated that no individual initiated flight before losing at least 11.5% body weight. The average weight loss necessary to initiate flight was 34%. In this study, 81.53% of all R. brethesi individuals exhibited flight behavior.
Knowledge about triatomine species that actively disperse by flight is useful for entomological surveillance programs in the Amazon. During an international meeting on the implementation of the intergovernmental initiative on the surveillance and prevention of Chagas disease in the Amazon, particular transmission situations were reported (including the involvement of the people working on the extraction of piassaba fibers) during the sylvatic cycle of T. cruzi in its natural ecotope. Increasing deforestation rates; the construction of houses closer to the forest where many palm tree species can be found; the presence of vectors, domestic and sylvatic reservoirs; and night lighting of households, which attracts the insects, all increase the risk of human contact with triatomine species in the Amazon.
CONCLUSION
The flight activity of R. brethesi was similar in the laboratory and in nature, as discussed in other studies that described reports of piassaba collectors being bitten by the species in its natural environment5,6,7.
According to the results of the experiments, R. brethesi usually disperses by flight between 11 and 15 days after the last blood meal, with a 34% weight loss from its initial weight occurring during this period. Moreover, R. brethesi required a minimum of 11% weight loss to initiate flight and meet its nutritional requirements. Increasing deforestation and the subsequent elimination of their usual food sources force the insects, which can be attracted by the presence of light in households close to deforested areas, to undergo a fasting period that leads them to disperse by flight after a week or two.
REFERENCES
1 Galvão C, Carcavallo RU, Rocha DS, Jurberg J. A checklist of the current valid species of the subfamily Triatominae Jeannel, 1919 (Hemíptera, Reduviidae) and their geographical distribution, with nomenclatural and taxonomic notes. Zootaxa. 2003;202:1-36. [Link]
2 Galvão C, Angulo VM. Belminus corredori, a new species of Bolboderini (Hemiptera: Reduviidae: Triatominae) from Santander, Colombia. Zootaxa. 2006;1241:61-8. [Link]
3 Schofield CJ, Galvão C. Classification, evolution, and species groups within the Triatominae. Acta Trop. 2009 May-Jun;110(2-3):88-100. [Link]
4 Poinar Jr G. Triatoma dominicana sp.n. (Hemiptera: Reduviidae: Triatominae), and Trypanosoma antiquus sp.n. (Stercoraria: Trypanosomatidae), the first fossil evidence of a triatomine-trypanosomatid vector association. Vector Borne Zoonotic Dis. 2005;5(1):72-81. Doi: 10.1089/vbz.2005.5.72 [Link]
5 Coura JR, Arboleda MN, Willcox HPF. Doença de Chagas na Amazônia brasileira. Rev SocBras Med Trop. 1993;26 Suppl 2:15-17.
6 Coura JR, Barreu TV, Arboleda MN. Ataque de populações humanas por triatomíneos silvestres no Amazonas: uma nova forma de transmissão da infecção chagásica? Soc Bras Med Trop. 1994 out-dez;27(4):251-4. [Link]
7 Coura JR, Arboleda MN, Willcox HPF. Chagas disease in the Brazilian Amazon. II-a serological survey. Rev Med Trop Sao Paulo. 1995 Mar-Apr,37(2):103-7. [Link]
8 Coura JR, Junqueira ACV, Boia MN, Fernandes O. Chagas disease: from bush to hus and houses. Is it the case of the Brazilian Amazon? Mem Inst Oswaldo Cruz. 1999;94(Suppl 1):378-84.
9 Miles MA. Human behaviour and the propagation of Chagas disease. Trans R Soc Trop Med Hyg. 1976;70(5-6):521-2. [Link]
10 Gamboa CJ. Dispersion de Rhodnius prolixus em Venezuela. Bol Dir Malariol San Amb. 1962;3:262-72.
11 Forattini OP, Ferreira AO, Rocha-Silva EO, Rabello EX, Santos JL. Aspectos ecológicos da tripanosomose americana. II - Distribuição e dispersão local de triatomíneos em ecótopos naturais e artificiais. Rev Saude Publica. 1971 dez;5(2):163-91. Doi:http://dx.doi.org/10.1590/S0034-89101971000200001 [Link]
12 Forattini OP, Rocha-Silva EO, Ferreira AO, Rabello EX, Patolli DGB. Aspectos ecológicos da tripanosomose americana. III - Dispersão local de triatomíneos, com especial referência ao Triatoma sórdida. Rev Saude Publica. 1971;5:193-205.
13 Ribeiro Jr G, Silva-Santos CG, Noireau F, Dias-Lima A. Potencial de dispersão de algumas espécies de Triatomíneos (Hemiptera: Reduviidae) por aves migratórias. Ser Ci Biol. 2006;6(4):324-8.
14 Abrahan LB, Gorla DE, Catalá SS. Dispersal of Triatoma infestans and other. Triatominae species in the arid Chaco of Argentina - Flying, walking or passive carriage? The importance of walking females. Mem Inst Oswaldo Cruz. 2011 Mar;106(2):232-9. [Link]
15 Lehane MJ, Mcewan PK, Whitaker CJ, Schofield CJ. The role of temperature and nutritional status in flight initiation by Triatoma infestans. Acta trop.1992 Sep;52(1):27-38.
16 Soares RPP, Santoro MM. α-glycerophosphate dehydrogenase activity in flight muscles of Triatominae bugs Panstrongylus megistus and Triatoma sordida. Mem Inst Oswaldo Cruz. 2000 Sep-Oct;95(5):707-9. [Link]
17 Mac CJR, Jurberg P, Lima MM. Marcação individual de triatomíneos para estudos comportamentais e ecológicos. Mem Inst Oswaldo Cruz. 1983 out-dez;78:473-6. [Link]
18 Galvão C, Rocha DS, Jurberg J, Carcavallo RU. Início da atividade de vôo em Triatoma infestans (Klug, 1834) e T. melanosoma Martínez, Olmedo & Carcavallo, 1987 (hemiptera, Reduviidae). Mem Inst Oswaldo Cruz. 2001 jan;96(1):137-40. [Link]
19 Rocha DS, Magalhães CS, Cunha V, Jurberg J, Galvão C. Ciclo biológico em laboratório de Rhodnius brethesi Matta, 1919 (Hemíptera, Reduviidae, Triatominae), potencial vetor silvestre da Doença de Chagas na Amazônia. Mem Inst Oswaldo Cruz. 2004 out,99(6):591-5. [Link]
20 Matta A. Sobre o gênero Rhodnius do Amazonas. Amazonas Med. 1922;5:161-2.
21 Mascarenhas BM. Triatomíneos da Amazônia. Sobre o ciclo evolutivo de Rhodnius brethesi Matta, 1919 (Hemíptera, Reduviidae, Triatominae). Bol Mus Par Emilio Goeldi Série Zol. 1990;6:191-202.
22 Mascarenhas BM. Triatomíneos da Amazônia: sobre o habitat e algumas considerações comportamentais de Rhodnius brethesi Matta, 1919 (Hemiptera, Reduviidae: Triatominae) na região do médio Rio Negro, Amazonas. Bol Mus Par Emilio Goeldi Série Zol. 1991;7(2):107-16. Doi:35400003048593.0020 [Link]
23 Silva IG, Silva HHG. Influência da temperatura na biologia de triatomíneos. X. Triatoma vitticeps Stal, 1859 (Hemiptera, Reduviidae). Rev Goiana Med. 1988; jan-jun.34(1/2):39-45. [Link]
24 Gurevitz JM, Ceballos LA, Kitron U, Gurtler RE. Flight initiation of Triatoma infestans (Hemiptera: Reduviidae) under natural climatic conditions. J Med Entomol. 2006 Mar;43(2):143-50. [Link]
25 Gurevitz JM, Kitron U, Gurtler RE. Flight muscle dimorphism and heterogeneity in flight initiation of field-collected Triatoma infestans (Hemiptera: Reduviidae) . J Med Entomol. 2007 Mar;44(2):186-91. [Link]
 Correspondência /Correspondence / Correspondencia:
Correspondência /Correspondence / Correspondencia:
Nelson Antonio Bailão Ribeiro
Alameda Gonçalo Duarte no 56
Bairro: Jurunas
CEP:66030-040
Belém-Pará-Brasil
E-mail:nelsonribeiro@iec.pa.gov.br
Recebido em / Received / Recibido en: 3/3/2011
Aceito em / Accepted / Aceito en: 28/6/11











 texto en
texto en 
 Curriculum ScienTI
Curriculum ScienTI
