Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Pan-Amazônica de Saúde
Print version ISSN 2176-6215On-line version ISSN 2176-6223
Rev Pan-Amaz Saude vol.2 no.1 Ananindeua Mar. 2011
http://dx.doi.org/10.5123/S2176-62232011000100012
CASE REPORT | RELATO DE CASO | RELATO DE CASO
Congenital Chagas disease due to acute maternal Trypanosoma cruzi infection transmitted by the oral route
Doença de Chagas congênita por infecção aguda maternal por Trypanosoma cruzi transmitida via oral
Enfermedad de Chagas congénita por infección aguda maternal por Trypanosoma cruzi transmitida vía oral
Ana Yecê das Neves PintoI; Vera da Costa ValenteI; Sebastião Aldo da Silva ValenteI; Amira Consuelo de Melo FigueirasII
ISeção de Parasitologia, Instituto Evandro Chagas/SVS/MS, Ananindeua, Pará, Brasil
IIUniversidade Federal do Pará, Belém, Pará, Brasil
Endereço para correspondência
Correspondence
Dirección para correspondencia
ABSTRACT
A family of four, including a 24-year-old female, presented to our laboratory in December 2006 with a prolonged febrile syndrome of unknown etiology. After extensive laboratory screening, acute Chagas disease was confirmed by positive T. cruzi blood culture, combined with clinical, epidemiological and serological findings. The young female, her parents and husband received a daily dose of benznidazole, but she developed serious drug intolerance and amenorrhea. Her treatment was interrupted by a confirmed pregnancy of about 12 weeks of gestational age. The child was born prematurely on April 18, 2007 with low weight and signs of respiratory distress syndrome. Diagnostic screening tests for congenital infections, including Chagas disease, were negative during the perinatal period. About four months after birth, clinical findings generated the following clinical indicators of congenital disease: convergent strabismus, microcephaly and delayed psychomotor development. Serological tests confirmed seroconversion, and magnetic resonance findings included cystic lesions and intracranial calcifications. The authors discuss the critical nature of this serious public health problem in the region and suggest necessary revisions to the recommended treatment for pregnant patients with acute Chagas disease.
Keywords: Chagas disease; Infectious disease Transmission, Vertica; Trypanocidal Agents.
RESUMO
Uma família de quatro pessoas, incluindo uma mulher de 24 anos de idade, apresentou-se em nosso laboratório em dezembro de 2006 com uma síndrome febril prolongada de etiologia desconhecida. Após uma triagem laboratorial extensa, foi confirmada, por meio de hemocultura positiva para T. cruzi, combinada com achados clínicos, epidemiológicos e sorológicos, a ocorrência de doença de Chagas aguda. A paciente, seus pais e marido receberam uma dose diária de Benzonidazol, porém ela desenvolveu intolerância severa à droga e amenorreia. Seu tratamento foi interrompido devido à confirmação de gravidez de cerca de 12 semanas de idade gestacional. A criança nasceu prematuramente em 18 de abril de 2007 com baixo peso e sinais de síndrome do desconforto respiratório. Testes de triagem diagnóstica para infecções congênitas, incluindo a doença de Chagas, resultaram negativos durante o período perinatal. Aproximadamente quatro meses após o nascimento, os achados clínicos forneceram os seguintes indicadores clínicos de doença congênita: esotropia, microcefalia e atraso no desenvolvimento psicomotor. Testes sorológicos confirmaram a soroconversão e a ressonância magnética apresentou lesões císticas e calcificações intracranianas. Os autores discutem a natureza crítica deste grave problema de saúde pública na região e sugerem revisões necessárias ao tratamento recomendado para pacientes grávidas com a doença de Chagas aguda.
Palavras-chaves: Doença de Chagas; Transmissão Vertical de Doença Infecciosa; Tripanossomicidas.
RESUMEN
Una familia de cuatro personas, incluyendo a una mujer de 24 años de edad, se presentó en nuestro laboratorio en diciembre de 2006 con un síndrome febril prolongado de etiología desconocida. Luego de una extensa selección y análisis de laboratorio, se confirmó, por medio de hemocultivo positivo para T. cruzi, combinado con hallazgos clínicos, epidemiológicos y serológicos, la ocurrencia de la enfermedad de Chagas aguda. La paciente, sus padres y marido recibieron una dosis diaria de benzonidazol, pero ella desarrolló intolerancia severa a la droga y amenorrea. Su tratamiento fue interrumpido debido a la confirmación del embarazo con cerca de 12 semanas de edad gestacional. El niño nació prematuramente el 18 de abril de 2007 con bajo peso y señales de síndrome de dificultad respiratoria. Pruebas de selección diagnóstica para infecciones congénitas, incluyendo la enfermedad de Chagas, resultaron negativas durante el período perinatal. Aproximadamente cuatro meses después del nacimiento, los hallazgos clínicos suministraron los siguientes indicadores clínicos de enfermedad congénita: esotropia, microcefalia y retraso en el desarrollo psicomotor. Pruebas serológicas confirmaron la seroconversión y la resonancia magnética presentó lesiones císticas y calcificaciones intracraneanas. Los autores discuten la naturaleza crítica de este grave problema de salud pública en la región y sugieren revisiones necesarias al tratamiento recomendado para pacientes embarazadas con la enfermedad de Chagas aguda.
Palabras clave: Enfermedad de Chagas; Transmisión Vertical de Enfermedad Infecciosa; Agentes Tripanocidas.
INTRODUCTION
Brazil has been certified as free of Triatoma infestans, the major vector of Chagas disease. The last seroprevalence survey of Chagas disease made in rural areas from Brazil shows low infection rates among children up to five years of age and demonstrated 0.025% of congenital transmission1.
Despite this achievement, continuous entomological surveillance should not be halted. Indeed, this paper presents evidence that direct or indirect transmission by other non-domiciliated vectors could be improved, mainly in the Amazon region. This region has well-established T. cruzi enzootic cycles and has suffered ecological imbalance as a result of major anthropogenic changes in the nineties. These multifactorial influences increase the focal risk of Chagas disease in the Amazon region, as previously described2,3,4.
This is the first report of congenital Chagas disease autochthonous from Amazon region, resulting from maternal acute infection acquired during a family-centered outbreak of oral transmission.
CASE PRESENTATION
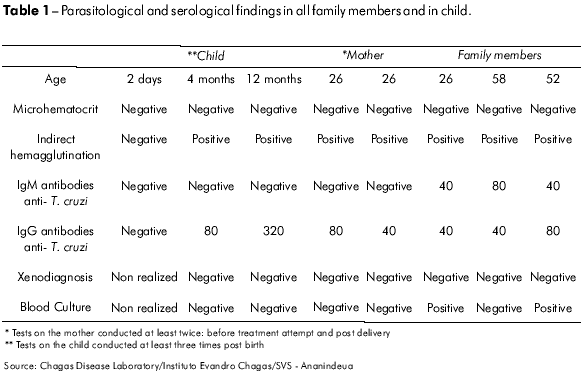
A family of four, including a young couple (male, 26 years old, and female, 24 years old) and her parents (52 and 56 years old) presented to Instituto Evandro Chagas (IEC), seeking a cure for their prolonged febrile illness. This disease appeared simultaneously in all four family members and was of approximately 18 days' duration. Diagnostics were conducted by two serological and three parasitological tests. Parasitological (positive T. cruzi blood culture in two of the patients), clinical and serological findings confirmed an acute Chagas disease outbreak in this family (Table 1).
Epidemiologic investigation did not find vectors associated with transmission in this family outbreak. In addition, the occurrence in an urban area and the almost simultaneous onset of symptoms in all four patients suggest an association between illness and a food (açaí juice) ingested by all four familiy members, which is not further discussed in this paper.
All patients received daily doses of benznidazole in January of 2007. The young woman showed serious drug intolerance with nausea, vomiting, abdominal pain and dizziness. Therefore, her treatment was interrupted due to suspected pregnancy (amenorrhea) and she experienced a spontaneous remission of symptoms. A pregnancy of 12 weeks' gestational age was confirmed, and the patient was followed clinically throughout the pregnancy. The patient's IgG anti-T. cruzi tests were consistently positive by Indirect Hemagglutination assay (IHA) and Indirect Immunofluorescent Assay Test (IFAT). Her serial parasitological tests (3) showed negative results. Throughout the pregnancy, the patient had no symptoms of Chagas disease. A morphological ultrasonograph from the 29th week of pregnancy showed no abnormalities.
The female child was born prematurely (34 weeks) on Apr. 18 2007, by cesarean section, with a low birth weight (1850 grs), jaundice and signs of respiratory distress syndrome (RDS), requiring intensive care. While in intensive care, she was diagnosed with bacterial pneumonia. Two days after birth, the newborn was submitted to parasitological and serological exams to detect T. cruzi infection by thick blood film, IFAT and IH. All of these test results were negative (Table 1).
The newborn recovered after three weeks and showed no alterations up to the fourth month of life. During a routine visit to our laboratory when the child had reached four months of age, we observed convergent strabismus and delayed psychomotor development (the child could not support her head). On physical examination, microcephaly and closed bregmatic suture were observed. Hematological parameters showed hemoglobin levels of 9.7 g%.
During this period, results of the search for T. cruzi in peripheral blood by the microhematocrit method, blood culture for T. cruzi, and indirect xenodiagnosis remained negative; however, we observed serological positive conversion by IH and IFAT (IgG anti – T. cruzi: 80). Table 1 shows all results from the mother and child at different times and the results from all persons studied in the course of this outbreak during the acute phase of Chagas disease. In order to evaluate other possible agents of congenital infection, a serologic study was conducted to detect the following: toxoplasmosis (IFAT); cytomegalovirs by the ELISA method; syphilis by the VDRL and FTA-Abs methods and rubella by the ELISA method. The results for all of these tests were negative in both mother and child, except for the test for IgG antibodies to cytomegalovirosis, which was positive in the mother.
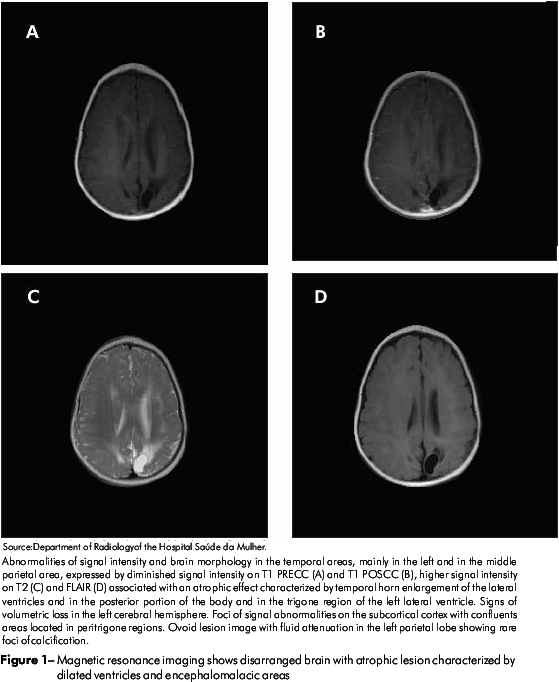
Magnetic resonance imaging showed abnormalities in the brain with atrophic lesions characterized by dilated ventricules and encephalomalacia areas, suggesting intracranial calcifications as well as cystic lesions (Figure 1).
The child was treated with benznidazole for 45 days and the case was reported to the Ananindeua Municipality Health Department. At 12 months of age, she remains under clinical observation. Her mother was treated, and the side effects of this treatment were rigorously controlled. The serological exams of both mother and daughter remained positive.
DISCUSSION
Studies conducted in T. cruzi-endemic areas found a Chagas disease prevalence of about 1% in pregnant women from Brazil, 4% to 6% in pregnant women from Argentina, 2% to 3% in pregnant women from Chile and 12% in pregnant women from Bolivia5. These rates are related to chronic maternal infection, in which the blood levels of parasites are low (1 to 10%)6. In contrast, during the acute phase, the blood parasite levels are high and induce the host immunological response. This state increases the risk of congenital infection7. However, the Brazilian Amazon is an area with active transmission of ACD and underestimated rates of chagasic infection8. These occurrences of ACD outbreaks in urban areas probably free of vectors and transmission by unusual form bring forward new epidemiological approaches to the control strategies of this ancient disease.
Moretti et al. (2005) described three pregnant women with ACD in Argentina. Two of them were infected during the third trimester and had uninfected newborns. One of the women became infected during the second trimester and gave birth to a sick child with hepatosplenomegaly9. As with any infection with the potential for vertical transmission, early pregnancy is associated with a high risk of infection. In this case, the mother's infection during the first trimester may have been a determinant of the child's infection and serious neurological involvement.
Studies carried out in Argentina, Bolivia, Paraguay and Brazil describe clinical spectra of congenital infection as well as asymptomatic to symptomatic forms. In symptomatic forms the clinical signs vary from mild to severe symptoms: premature births, low birth weight, hepatomegaly, splenomegaly, neurologic signs, jaundice, anemia, anasarca and RDS are commonly described10,11,12,13. In the child of the Amazon, the main clinical features were jaundice, anemia and RDS; however, low neurological development with intracranial calcifications is rarely described in congenital Chagas disease. Only one report from La Paz, Bolívia is similar to the one described here14. On the other hand, another series of cases in Argentina shows that 64.8% of children lacked clinical signs and the hepatomegaly was the main clinical sign in symptomatic children12.
We were unable to find parasites in the child's peripheral blood at any point during the follow-up. The diagnosis was accomplished by serological conversion tests, epidemiological data (data related to mother-to-child transmission from a mother with ACD) and clinical findings. The sensibility of parasitological tests is reduced in congenital cases due to low parasitaemias and the diagnosis is frequently realized by serology. Thus, the clinical follow-up of a child plays an important role in diagnosis and, subsequently, in the potential response (negative serology) to treatment.
Rigorous control of ACD in pregnant women and their newborns from high-risk areas of the Amazon during the prenatal and perinatal periods poses an important challenge to public health surveillance systems. Mothers with positive serological reactions should, together with their newborns, undergo direct parasitological exams by microhematocrit immediately after birth. In addition, these children must be followed at least up to nine months of age. In Brazil, the consensus of experts reinforces the serological screening of pregnants and follow-up of children born from chagasic mothers15,16. Nevertheless, this important recommendation is frequently neglected in Brazil and in most of the endemic countries.
The second challenge in the control of Chagas disease is the revision of treatment protocols for administering benznidazole to pregnant women with ACD. Pregnant women may need close, individualized care to use benznidazole safely17. We suggest that, especially after the twenty-fourth week of pregnancy (when the potential drug harm to fetus is diminished), the indication for benznidazole could be discussed with the mother. Mainly, the development of effective drugs with minimal side effects must be a priority of Public Health Systems in areas with active foci of Chagas disease transmission.
Moreover, all efforts should be made to strengthen the health surveillance systems to Chagas disease in Brazil. Therefore, capability of human resources is the main control strategy to enhance the quality of laboratory diagnosis and to improve the sensibility of clinical suspicion at individual primary care level, as well as to prepare specialized personnel with sustainable performance to understand the complexity of transmission cycles components of this ancient public health problem in Brazil, but emerging in Amazon region.
CONCLUSIONS
In pregnant women with ACD, there is an increased risk of disease transmission to the fetus compared to mothers with chronic infection. In addition, the potential for irreversible lesions in the newborns as a result of this vertical transmission (as found in this report), suggests that it is necessary to revise policy recommendations on the use of trypanocidal drugs in these cases.
ACKNOWLEDGMENTS
we thank Francelino de Almeida Araújo Júnior from Saúde da Mulher Hospital, Belém, for RM images.
FINANCIAL SUPPORT
Instituto Evandro Chagas and UNESCO
REFERÊNCIAS
1 Ostermayer AL, Passos ADC, Silveira AC, Ferreria AW, Macedo V, Prata A. O inquérito nacional de soroprevalência de avaliação do controle da doença de Chagas no Brasil (2001-2008). Rev Soc Bras Med Trop. 2011;44 Suppl 2:S108-21. [Link]
2 Coura JR, Junqueira AC, Fernandes O, Valente SA, Miles MA. Emerging Chagas disease in Amazonian Brazil. Trends Parasitol. 2002 Apr;18(4):171-6. Doi:doi:10.1016/S1471-4922(01)02200-0 [Link]
3 Pinto AYN, Ferreira Jr AG, Valente VC, Harada GS, Valente SAS. Urban Outbreak of Emerging acute Trypanosomiasis in Brazilian Amazon: 4-year follow-up post treatment with benznidazol. Rev Panam Salud Publica. 2009 Jan;25(1):77-83.
4 Valente SA, Valente VC, Pinto AYN. Epidemiologia e transmissão oral da doença de Chagas na Amazônia brasileira. In: Organización Panamericana de la Salud. Organización Mundial de la Salud. Unidad. Informe de la consulta técnica en Epidemiología, Prevención y Manejo de la Transmisión de la Enfermedad de Chagas como Enfermedad Transmitida por Alimentos. Rio de Janeiro: OPS/OMS; 2006. p. 21-6. [Link]
5 Schmunis GA. A Tripanosomíase Americana e seu impacto na Saúde Pública das Américas. In: Brener Z, Andrade ZA, Barral-Neto M, editors. Trypanosoma cruzi e Doença de Chagas. 2. ed. Rio de Janeiro: Guanabara Koogan; 2000. p. 1-15.
6 Bittencourt AL. Transmissão vertical da doença de Chagas. In: Brener Z, Andrade ZA, Barral-Neto M, editors. Trypanosoma cruzi e doença de Chagas. 2. ed. Rio de Janeiro: Guanabara Koogan; 2000. p. 16-20.
7 Hermann E, Truyens C, Alonso-Vega C, Rodriguez P, Berthe A, Torrico F, et al. Congenital transmission of Trypanosoma cruzi is associated with maternal enhanced parasitemia and decreased production of interferon- gamma in response to parasite antigens. J Infect Dis. 2004 Apr;189(7):1274-81. [Link]
8 Organização Pan Americana da Saúde. Guia para vigilância, prevenção, controle e manejo clínico da doença de Chagas aguda transmitida por alimentos. Rio de Janeiro: PANAFTOSA-VP/OPAS/OMS; 2009. p. 92. (Serie de Manuais Técnicos, 12). [Link]
9 Moretti E, Basso B, Castro I, Carrizo Paez M, Chaul M, Barbieri G, et al. Chagas disease: study of congenital transmission in cases of acute maternal infection. Rev Soc Bras Med Trop. 2005 Jan-Feb;38(1):53-5. [Link]
10 Torrico F, Alonso-Vega C, Suarez E, Rodríguez P, Torrico MC, Dramaix M, et al. [Endemic level of congenital Trypanosoma cruzi infection in the areas of maternal residence and the development of congenital Chagas disease in Bolivia]. Rev Soc Bras Med Trop. 2005;38 Suppl 2:17-20. [Link]
11 Negrette OS, Mora MC, Basombrio MA. High prevalence of Congenital Trypanosoma cruzi infection and family clustering in Salta, Argentina. Pediatrics 2005 Jun; 115(6):668-72. [Link]
12 Freilij H, Altched J. Congenital Chagas' disease: diagnostic and clinical aspects. Clin Infect Dis. 1995 Sep;21(3):551-5. [Link]
13 Gürtler ER, Segura E, Cohen J. Congenital transmission of Trypanosoma cruzi infection in Argentina. Emerg Inf dis. 2003 Jan;9(1):29-32. [Link]
14 Pehrson P, Walgren M, Bengstsson E. Intracranial calcifications probably due to congenital chagas disease. Am J Trop Med Hyg. 1982 May;31(3):449-51.
15 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Consenso Brasileiro em Doença de Chagas. Rev Soc Bras Med Trop. 2005;38 Suppl 3:S30.[Link]
16 Gontijo ED, Andrade GMQ, Santos SE, Galvã LMC, Moreira EF, Pinto FS, et al. Triagem neonatal da infecção pelo Trypanosoma cruzi em Minas Gerais, Brasil: transmissão congênita e mapeamento das áreas endêmicas. Epidemiol Serv Saúde. 2009 jul-set;18(3):243-54. [Link]
17 Bittencourt AL. Actual aspects and epidemiological significance of congenital transmission of Chagas' disease. Mem Inst Oswaldo Cruz. 1984;79 Suppl:133-7. [Link]
 Correspondence / Correspondência / Correspondencia:
Correspondence / Correspondência / Correspondencia:
Ana Yecê das Neves Pinto,
Setor Médico Unificado (SOAMU)/Epidemiologia,
Instituto Evandro Chagas/SVS/MS
BR 316 Km 7 s/n,
ZIP code: 67030-070, Ananindeua/PA
Phone – FAX number: 91 32142189
E.mail:ayece@iec.pa.gov.br
Recebido em / Received / Recibido en: 10/5/2011
Aceito em / Accepted / Aceito en: 18/7/2011











 text in
text in 
 Curriculum ScienTI
Curriculum ScienTI
