Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Pan-Amazônica de Saúde
versão impressa ISSN 2176-6215versão On-line ISSN 2176-6223
Rev Pan-Amaz Saude v.3 n.1 Ananindeua mar. 2012
http://dx.doi.org/10.5123/S2176-62232012000100003
ORIGINAL ARTICLE | ARTIGO ORIGINAL | ARTÍCULO ORIGINAL
Eggshell as a characteristic to identify Lutzomyia (Nyssomyia) intermedia (Lutz & Neiva, 1912) and Lutzomyia (Nyssomyia) neivai (Pinto, 1926) (Diptera: Psychodidae: Phlebotominae), vectors of cutaneous leishmaniasis
Cascas de ovos como um fator de identificação de Lutzomyia (Nyssomyia) intermedia (Lutz & Neiva, 1912) e Lutzomyia (Nyssomyia) neivai (Pinto, 1926) (Diptera: Psychodidae: Phlebotominae), vetores da leishmaniose tegumentar
Cascaras de huevos como un factor de identificación de Lutzomyia (Nyssomyia) intermedia (Lutz & Neiva, 1912) y Lutzomyia (Nyssomyia) neivai (Pinto, 1926) (Diptera: Psychodidae: Phlebotominae), vectores de la leishmaniasis tegumentaria
Wagner Alexandre CostaI; Simone Miranda da CostaI; Elizabeth Ferreira RangelI; Jacenir Reis dos Santos-MalletI; José Eduardo SerrãoII
ILaboratório de Transmissores de Leishmanioses, Instituto Oswaldo Cruz, Rio de Janeiro, Rio de Janeiro, Brasil
IIDepartamento de Biologia Geral, Universidade Federal de Viçosa, Viçosa, Minas Gerais, Brasil
Endereço para correspondência
Correspondence
Dirección para correspondencia
ABSTRACT
Sand flies are the main vectors of leishmaniasis diseases that infect approximately 12 million people in 88 countries, with approximately 350 million people living in risk areas. Sand fly species are identified based on the morphology of the adults, and there are few studies of the immature insects, making the accurate identification of these vectors prior to adult emergence difficult. This study investigated the eggshell morphology of Lutzomyia (Nyssomyia) intermedia and Lutzomyia (Nyssomyia) neivai. The results obtained with scanning electron microscopy and quantitative analyses showed the occurrence of significant differences among numbers of tubercles in the eggshell of both species, besides the presence of some cross-conections between ridges, only in L. (N.) intermedia, thus enabling the morphological differentiation between eggs of both species.
Keywords: Psychodidae; Insect Vectors; Animal Structure; Scanning Electron Microscopy; Chorionic Sculpturing; Systematics.
RESUMO
Os flebotomíneos são os principais vetores das leishmanioses. Essas doenças atingem aproximadamente 12 milhões de pessoas em 88 países, e cerca de 350 milhões de pessoas vivem em áreas de risco. As espécies de flebotomíneos são identificadas com base na morfologia de seus espécimes adultos. A existência de poucas pesquisas sobre espécimes imaturos torna difícil uma identificação exata desses vetores antes de atingirem a idade adulta. Este estudo investigou a morfologia das cascas de ovos das espécies Lutzomyia (Nyssomyia) intermedia e Lutzomyia (Nyssomyia) neivai. Os resultados obtidos por meio de microscopia eletrônica e análises quantitativas demonstraram a ocorrência de diferenças significativas entre os números de tubérculos nas cascas de ovos de ambas as espécies, além da presença de algumas conexões cruzadas entre cristas apenas nos espécimes de L. (N.) intermedia, possibilitando assim a diferenciação morfológica de seus ovos.
Palavras-chave: Psychodidae; Insetos Vetores; Estrutura Animal; Microscopia Eletrônica de Varredura; Escultura Coriônica; Sistemática.
RESUMEN
Los flebótomos son los principales vectores de las leishmaniasis. Esas enfermedades alcanzan a aproximadamente 12 millones de personas en 88 países, y cerca de 350 millones de personas viven en áreas de riesgo. Las especies de flebótomos se identifican con base en la morfología de sus especímenes adultos. La existencia de pocas investigaciones sobre especímenes inmaduros torna difícil una identificación exacta de esos vectores antes de alcanzar la edad adulta. Este estudio investigó la morfología de las cascaras de huevos de las especies Lutzomyia (Nyssomyia) intermedia y Lutzomyia (Nyssomyia) neivai. Los resultados obtenidos a través de microscopia electrónica y análisis cuantitativos demostraron la ocurrencia de diferencias significativas entre los números de tubérculos en las cascaras de huevos de ambas especies, además de la presencia de algunas conexiones cruzadas entre cristas apenas en los especímenes de L. (N.) intermedia, posibilitando así la diferenciación morfológica de sus huevos.
Palabras clave: Psychodidae; Insectos Vectores; Estructura Animal; Microscopía Electrónica de Barridoa; Escultura Coriónica; Sistemático.
INTRODUCTION
Sand flies are important to public health because they are the main vectors of leishmaniasis diseases, including American cutaneous leishmaniasis (ACL), which affects 25,500 individuals/year in Brazil1. The disease has different clinical and pathological features due to the diversity of the parasites and the immunity of the infected individuals2,3,4. To date, 45 species of sand flies are estimated to be associated with the transmission of cutaneous leishmaniasis in the New World5,6.
Sand fly species are identified based on the morphology of the adult insects, and there are few studies of the immature stages. The immature stages of only approximately 70 species of the 510 described species of Neotropical sand flies have been well characterized7,8,9,10,11,12.
Lutzomyia (Nyssomyia) intermedia and Lutzomyia (Nyssomyia) neivai are the main vectors of dermal leishmaniasis in Southern and Southeastern Brazil4,5,13. L. (N.) intermedia was described by Lutz & Neiva (1912) using adult specimens collected in Ouro Fino farm, in the City of Além Paraíba, Minas Gerais State. L. (N.) intermedia is considered as the first species described in the Neotropics and was redescribed in 1996 by Marcondes14. L. (N.) neivai was described by Pinto (1926) using a male trapped inside a residence at the Instituto Butantan, in the City of São Paulo, São Paulo State. These species may occur together in the same place, especially in the Vale da Ribeira, São Paulo State. For several years, these two species were synonyms; however, morphological studies of different populations of L. (N.) intermedia in Brazil, showed that this species could be a species complex of L. (N.) intermedia sensu strictu together with L. (N.) neivai. The former species is found in regions of low altitude, and the second is adapted to cold and dry environments14,15,16.
Although both species show some degree of genetic introgression, revisited L. (N.) intermedia and L. (N.) neivai were concluded to be valid species based on the following characteristics: the maximum width of the spermathecal rings, symmetry of annulation, head shape, length of the spermathecal duct and shape of the male genital filaments15,17.
The advent of taxonomic tools based on such technological advances as scanning electron microscopy (SEM) since 1 965 has been useful to clarify the taxonomic status of some species complexes18,19,20,21,22. Thus, the characterization of immature stages of insects using highresolution tools may contribute to the accurate identification of sand fly species. This study investigated the eggshell ornamentation of the eggs of L. (N.) intermedia and L. (N.) neivai, showing that the eggs can be used to identify both of the species.
MATERIALS AND METHODS
INSECTS
The adult specimens were collected from areas with an occurrence of leishmaniasis in Southeast Brazil. L. (N.) intermedia was collected in Jacarepaguá, a Municipality of Rio de Janeiro, Rio de Janeiro State, and in the Municipality of Iporanga, São Paulo State. L. (N.) neivai was collected in the Planalto region of São Paulo State. Both species were collected using HP-type light traps23.
Colonies of these two species were maintained in the Laboratório de Transmissores de Leishmanioses, Instituto Oswaldo Cruz and in the Escola de Saúde Pública, Universidade de São Paulo following described procedures24,25.
SCANNING ELECTRON MICROSCOPY
A total of 80 eggs of each species was obtained from the colonies and transferred to a 2.5% glutaraldehyde solution in 0.1 M sodium cacodylate buffer (pH 7.2) for 1 h. The eggs were then post-fixed in 1% osmium tetroxide in the same buffer for 1 h at room temperature. After washing in the same buffer, the eggs were dehydrated in a graded ethanol series, dried in a critical point dryer with CO2 (Balzers), covered with gold (20 nm) and analyzed using a LEO 1430 VP scanning electron microscope (SEM) at the Centro de Microscopia e Microanálise at the Universidade Federal de Viçosa, Minas Gerais State, Brazil, and using a Jeol 6390 LV in the Rudolf Barth Electron Microcopy Platform at Fundação Oswaldo Cruz, Rio de Janeiro State, Brazil.
MORPHOMETRY
The SEM images were analyzed using the computer program SemAfore 3.0 (JEOL, Sollentuna, Sweden), and the number of eggshell tubercles was calculated considering a 1 µm2 area/egg.
STATISTICAL ANALYSIS
The ANOVA test was applied at a significance level of 0.05 using SPSS 10.0 for Windows (SPSS Inc., 233 South Wacker Drive, 11th Floor, Chicago, IL).
RESULTS
The eggs of L. (N.) intermedia and L. (N.) neivai were dark, elliptical, elongated and slightly flattened. The anterior and posterior regions of the eggs of both species were rounded, presenting two round aeropylar apertures at the end (Figures 1D, 2B and 2D). The L. (N.) intermedia eggs measured 361 µm in length and 75.4 µm in width in their central region; the L. (N.) neivai eggs measured 343 µm in length and 85.5 µm in width (Figures 1A and 2A).
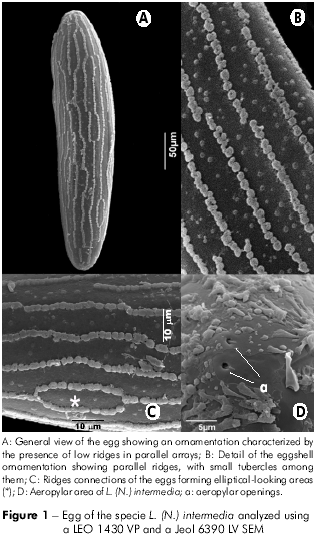
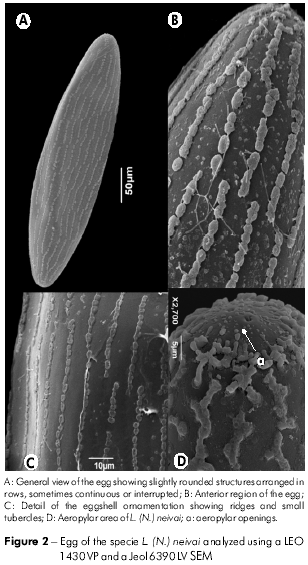
The exochorion showed an ornamentation characterized by the presence of low ridges, with parallel arrays along the long axis of the egg (Figures 1A and 2A), which were formed by slightly rounded structures arranged in rows that were continuous or interrupted (Figures 1B and 2C). The ridges had no cross-connections between them on the L. (N.) neivai eggs (Figures 2B and 2C), whereas some connections were observed on the L. (N.) intermedia eggs, forming elliptical-looking areas (Figure 1C). Small isolated tubercles were found between the spaces formed by the ridges (Figures 1B and 2B), varying in number between the two studied species: 8.55 ± 1.80 tubercles/1 µm2 in L. (N.) intermedia and 5.95 ± 1.87 tubercles/1 µm2 in L. (N.) neivai. When applying ANOVA, a difference was observed between the number of tubercles per 1 µm2 (0,05 < p; p = 0,0).
DISCUSSION
Several studies investigating the surfaces of eggs have been conducted in Diptera with regard to taxonomic identification and oviposition sites26,27,28,29,30,31. SEM is a powerful tool that complements classical taxonomy, as many structures that are not visible using light microscopy may be important for the morphological characterization of insects32,33,34,35.
The occurrence of structures on the L. (N.) intermedia and L. (N.) neivai exochorion characterized by the occurrence of longitudinal ridges without connections between each other is a character commonly found in sand flies. A study of five sand fly species reported parallel ridges forming polygons at irregular intervals in L. caballeroi or connected ridges and a reticular pattern in L. peruensis. Some of these ridges are subdivided to form elliptical or hexagonal areas20,22, similar to our observations on some regions of the chorion of L. (N.) intermedia. In contrast, the authors of the above study observed an undefined pattern in L. tejedai. Our results show that it is possible to identify L. (N.) intermedia and L. (N.) neivai based on the eggshell morphology, similar to the sand flies Phlebotomus and Sergentomyia36, corroborating that the ornamentation of the exochorion is a good characteristic for species identification in Diptera27,28.
Considering the origin and function of the exochorion, some authors have suggested the existence of a relationship between the species and their larval development in micro-ecosystems and that the eggshell ornamentations are adaptive structures that facilitate oviposition in different environments18,20. The chorion is secreted by the ovarian follicular epithelial cells during choriogenesis, and its viscosity allows the egg to adhere to the substrate. The chorion is a rigid structure forming the eggshell and is composed of two layers: an inner layer, the endochorion in contact with the embryo, and an outer layer, the exochorion in contact with the environment36. When desiccated, the chorion presents distinct layers, generally forming elaborate structures on the surface of the egg, with the patterns being characteristic for each species. This layer serves to cover and protect the embryo, preventing it from desiccation and regulating gaseous exchange37,21,38. Since L. (N.) intermedia is found in warm and humid environments, whereas L. (N.) neivai is found in cold and dry environments possible association between differences on egg surface of both species to enviroments should be evaluated. In addition to studies on the ecological adaptations, the eggshell structures of these species may contribute to future investigations of the phylogenetic relationships of the genus Lutzomyia, as suggested for other representative sand flies37,19,20,21,38.
CONCLUSION
The study of the immature insect forms by SEM produces a deeper knowledge of the different species of sandflies, with the purpose of giving a precise identification based on the immature stages.
Our findings using scanning electron microscopy and the quantitative analysis of the eggshell tubercles showed a variation of this structure that enables a morphological differentiation between L. (N.) intermedia and L. (N.) neivai.
ACKNOWLEDGMENTS
The authors thank the Centro de Microscopia e Microanálise at the Universidade Federal de Viçosa for technical assistance; Dr. Claudio Casanova of the Superintendência para Controle de Doenças Endêmicas de São Paulo; Dr. Eunice Aparecida Bianchi Galati and Ms. Edna Maria Fátima Bueno of the Departamento de Epidemiologia at the Escola de Saúde Pública; Universidade de São Paulo for the donation of the eggs; Dr. Alfredo Azevedo for his assistance in statistical analysis and Dr. Simone Patricia Carneiro de Freitas, Mr. William Marques de Almeida and Mr. José Adalberto da Silva of the Laboratório de Transmissores de Leishmanioses, Instituto Oswaldo Cruz/FIOCRUZ for their technical assistance.
FINANCIAL SUPPORT
This research was supported by Fundação de Amparo à Pesquisa do Estado de Minas Gerais and Conselho Nacional de Desenvolvimento Científico e Tecnológico.
REFERENCES
1 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Coordenação Geral de Doenças Transmissíveis. Coordenação Gerência Técnica das Leishmanioses. Brasília: Ministério da Saúde; 2011.
2 Lainson R, Shaw JJ. Epidemiology and ecology of leishmaniasis in Latin-America. Nature. 1978 Jun;273(5664):595-600. [Link]
3 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Manual de vigilância e controle da Leishmaniose visceral. Brasília: Mistério da Saúde; 2003. 122 p. (Série A. Normas e Manuais Técnicos).
4 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Manual de Vigilância da Leishmaniose Tegumentar Americana. 2. ed. Brasília: Ministério da Saúde; 2007. 182 p. (Serie A. Normas e Manuais Técnicos).
5 Rangel EF, Laison R. Proven and putative vectors of American cutaneous leishmaniasis in Brazil: aspects of their biology and vectorial competence. Mem Inst Oswaldo Cruz. 2009 Nov;104(7):937-54. http://dx.doi.org/10.1590/S0074-02762009000700001 [Link]
6 World Health Organization. Leishmaniasis [Internet]. Geneva. (cited 2012 Jul 4). Available from: http://www.who.int/leishmaniasis/en/.
7 Andrade-Filho JD. Morfologia do Complexo Nyssomyia intermedia (Diptera: Psychodidae: Phlebotominae). [dissertação]. São Paulo (SP): Universidade de São Paulo, Faculdade de Saúde Pública; 2003.
8 Araki AS, Vigoder FM, Bauzer LG, Ferreira GE, Souza NA, Araújo IB, et al. Molecular and behavioral differentiation among Brazilian populations of Lutzomyia longipalpis (Diptera: Psychodidae: Phlebotominae). PLoS Negl Trop Dis. 2009;3(1):e365. Doi:10.1371/journal.pntd.0000365 [Link]
9 Bauzer LGSR, Gesto JSM, Souza NA, Ward RD, Hamilton JGC, Kyriacou CP, et al. Molecular divergence in the period gene between two putative sympatric species of the Lutzomyia longipalpis complex. Mol Biol Evol. 2002 Sep;19(9):1624-7. [Link]
10 Bauzer LG, Souza NA, Maingon RD, Peixoto AA. Lutzomyia longipalpis in Brazil: a complex or a single species? A mini-review. Mem Inst Oswaldo Cruz. 2007 Feb;102(1):1-12.
11 Ready PD, Rangel EF. Caracteres isoenzimáticos e moleculares: espécies crípticas. In: Rangel EF, Lainson R, editores. Flebotomíneos do Brasil. Rio de Janeiro: FIOCRUZ; 2003. p. 185-206. [Link]
12 Vigoder FM, Souza NA, Peixoto AA. Acoustic signals in the sand fly Lutzomyia (Nyssomyia) intermedia (Diptera: Psychodidae). Parasit Vectors. 2011 May;4:76. Doi:10.1186/1756-3305-4-76 [Link]
13 Pita-Pereira D, Souza GD, Zwetsch A, Alves CR, Brito C, Rangel EF. First report of Lutzomyia (Nyssomyia) neivai (Diptera: Psychodidae: Phlebotominae) naturally infected by Leishmania (V.) braziliensis in a periurban area of South Brazil, State of Rio Grande do Sul, using a PCR multiplex assay. Am J Trop Med Hyg. 2009 Apr;80(4):593-5.
14 Marcondes CB. A redescription of Lutzomyia (Nyssomyia) intermedia (Lutz & Neiva, 1912) and resurrection of L. neivai (Pinto 1926) (Diptera: Psychodidae: Phlebotominae). Mem Inst Oswaldo Cruz. 1996 Jul-Aug;91(4):457-62. http://dx.doi.org/10.1590/S0074-02761996000400012 [Link]
15 Andrade Filho JDA, Galati EAB, Falcão AL. Nyssomyia intermedia (Lutz & Neiva, 1912) and Nyssomyia neivai (Pinto, 1926) (Diptera: Psychodidae: Phlebotominae) geographical distribution and epidemiological importance. Mem Inst Oswaldo Cruz. 2007 Jun;102(4):481-7. [Link]
16 Marcondes CB, Lozovei AL, Vilela JH. Distribuição geográfica de flebotomíneos do complexo Lutzomyia intermedia (Lutz & N eiva , 1912) (Diptera , Psychodidae). Rev Soc Bras Med Trop. 1998 jan-fev;31(1):51-8.
17 Marcondes CB. Morfometria e DNA mitochondrial de populações sul americanas de Lutzomyia (Nyssomyia) intermedia (Lutz & N eiva, 1912) (Diptera: Psychodidae: Phlebotominae) [tese]. Curitiba (PR): Universidade Federal do Paraná, Departamento de Ciências Biológicas; 1997.
18 Ward RD, Ready PA. Chorioic sculpturing in some sandfly eggs (Diptera: Psychodidae). J Entomol. 1975 Jul;50(2):127-34. DOI:10.1111/j.1365-3032.1975.tb00101.x [Link]
19 Feliciangeli MD, Castejon OC, Limongi J. Egg surface ultrastructure of eight new world phlebotomine sandfly species (Diptera: Psychodidae). J Med Entomol. 1993 Jul;30(4):651-6. [Link]
20 Perez JE, Ogusuku E. Chorion pattern on eggs of Lutzomyia sandflies from the Peruvian Andes. Med Vet Entomol. 1997 Apr;11(2):127-33. DOI:10.1111/j.1365-2915.1997.tb00301.x [Link]
21 Fausto AM, Feliciangeli MD, Maroli M, Mazzini M. Ootaxonomic investigation of five Lutzomyia species (Diptera, Psychodidae) from Venezuela. Mem Inst Oswaldo Cruz. 2001 Feb;96(2):197-204. http://dx.doi.org/10.1590/S0074-02762001000200011 [Link]
22 Almeida DN, Oliveira RS, Brazil BG, Soares MJ. Patterns of exochorion ornaments on eggs of seven South American species of Lutzomyia sand flies (Diptera: Psychodidae). J Med Entomol. 2004 Sep;41(5):819-25. http://dx.doi.org/10.1603/0022-2585-41.5.819 [Link]
23 Pugeto H, Barata RA, França-Silva JC, Silva JC, Dias ES. HP: an improved modelo of sucction light trap for the capture of small insects. Rev Soc Bras Med Trop. 2005 Jan-Feb;38 (1):70-2. [Link]
24 Modi GB, Tesh RB. A simple technique for mass rearing Lutzomyia longipalpis and Phlebotomus papatasi (Diptera: Psychodidae) in the laboratory. J Med Entomol. 1983 Oct;20(5):568-9.
25 Rangel EF, Souza NA, Wermelinger ED, Barbosa AF. Estabelecimento de colônia, em laboratório, de Lutzomyia intermedia Lutz & Neiva, 1912 (Diptera, Psychodidae, Phlebotominae). Mem Inst Oswaldo Cruz. 1985 abr-jun;80(2):219-26. [Link]
26 Choochote W, Jitpakdi A, Sukontason K, Suntaravitun T, Wongkamchai S, Sukontason K, et al. Scanning electron microscopy of Aedes lineatopennis (Diptera: Culicidae) Eggs. J Med Entomol. 2001 Sep;38(5):753-5. http://dx.doi.org/10.1603/0022-2585-38.5.753 [Link]
27 Alencar J, Guimarães AE, Mello RP, Lopes CM, Dégallier N, Santos-Mallet JR. Scanning electron microscopy of eggs of Hemagogus leucocelaenus (Diptera: Culicidae). Rev Saude Publica. 2003 Oct;37(5):658-61. http://dx.doi.org/10.1590/S0034-89102003000500017 [Link]
28 Alencar J, Guimarães AE, Gil-Santana HR, Santos-Mallet JR. Scanning electron microscopy of eggs of Ochlerotatus (Protomacleaya) terrens Walker. J Am Mosq Control Assoc. 2005 Dec;21(4):355-9. [Link]
29 Mendonça PM, Santos-Mallet JR, Mello RP, Gomes L, Queiroz MMC. Identification of fly eggs using scanning electron microscopy for forensic investigations. Micron. 2008 Oct;39(7):802-7. [Link]
30 Santos-Mallet JR, Gleiser RM, Alencar J, Marques WA, Sarmento JS, Muller GA, et al. Scanning electron microscopy of the eggs of Ochlerotatus albifasciatus (Diptera: Culicidae). J Med Entomol. 2009 Sep;46(5):980-5. http://dx.doi.org/10.1603/033.046.0502 [Link]
31 Santos-Malet JR, Muller GA, Gleiser RM, Alencar J, Marques WA, Sarmento JS. Scanning electron microscopy of the eggs of Aedes scapularis from southern South America. J Am Mosq Control Assoc. 2010 Jun;26(2):205-9. http://dx.doi.org/10.2987/09-5959.1 [Link]
32 Serrão JE. A comparative study of the proventricular structure in corbiculate apinae (Hymenoptera, Apidae). Micron. 2001 Jun;32(4):379-85. http://dx.doi.org/10.1016/S0968-4328(00)00014-7 [Link]
33 Serrão JE. Proventricular structure in the solitary bees (Hymenoptera, Apidae). Org Divers Evol. 2005 Jun;5(2):125-33. http://dx.doi.org/10.1016/j.ode.2004.10.011 [Link]
34 Serrão JE. Proventricular structure in the bee tribe Augochlorini (Hymenoptera, Halictidae). Org Divers Evol. 2007 Nov;7(3):175-80. http://dx.doi.org/10.1016/j.ode.2006.05.002 [Link]
35 Szinwelski N, Rodrigues MS, Pereira MR, Serrão JE, Sperber CF. Proventriculus of three nemobiinae crickets (Orthoptera: Grylloidea: Trigonidiidae). J Orthoptera Res. 2009;18(1):59-63.
36 Perfil'iev PP. Phlebotominae (sandflies). In: Fauna of the USSR. Israel Program for of Scientific Translations. Jerusalem: Academy of Sciences of USSR; 1968. 362 p.
37 Endris RG, Young DG, Perkins PV. Ultrastructural comparison of egg surface morphology of five Lutzomyia species (Diptera: Psychodidae). J Med Entomol. 1987 Jul;24(4):412-5.
38 Bahia AC, Secundino NFC, Miranda JC, Prates DB, Souza APA, Fernandes FF, et al. Ultrastructural comparison of external morphology of immature stages of Lutzomyia (Nyssomyia) intermedia and Lutzomyia (Nyssomyia) whitmani (Diptera: Psychodidae), vectors of cutaneous leishmaniasis, by scanning electron microscopy. J Med Entomol. 2007 Nov;44(6):903-14. http://dx.doi.org/10.1603/0022-2585(2007)44[903:UCOEMO]2.0.CO;2 [Link]
 Correspondence / Correspondência /Correspondencia:
Correspondence / Correspondência /Correspondencia:
Jacenir Reis dos Santos-Mallet
Av. Brasil, 4365. Bairro: Manguinhos
CEP: 21040-360
Rio de Janeiro-Rio de Janeiro-Brasil
Tel.: +55 (21) 2562-1352
E-mail: ¡acenir@ioc.fiocruz.br
Received / Recebido em / Recibido en: 23/3/2012
Accepted / Aceito em / Aceito en: 20/8/2012













