Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Pan-Amazônica de Saúde
versão impressa ISSN 2176-6215versão On-line ISSN 2176-6223
Rev Pan-Amaz Saude v.3 n.2 Ananindeua jun. 2012
http://dx.doi.org/10.5123/S2176-62232012000200002
ORIGINAL ARTICLE |ARTIGO ORIGINAL | ARTÍCULO ORIGINAL
Distribution of HIV-1 subtypes in patients with HAART therapeutic failure in the States of Pará and Amazonas, Brazil: 2002 to 2006
Distribuição de subtipos de HIV-1 em pacientes com falha terapêutica à HAART nos Estados do Pará e Amazonas, Brasil, entre 2002 e 2006
Distribución de subtipos de VIH-1 en pacientes con fallo terapéutico al HAART en los Estados de Pará y Amazonas, Brasil, entre 2002 y 2006
Olinda MacêdoI; Luciana Macêdo FerreiraII; Carmen Andréa Freitas Lopes III; Rita Catarina Medeiros de SousaIV; José Ricardo Mourão AraújoV; Pedro Fernando da Costa VasconcelosII;
ISeção de Virologia, Instituto Evandro Chagas/SVS/MS, Ananindeua, Pará, Brasil
IISeção de Arbovirologia e Febres Hemorrágicas, Instituto Evandro Chagas/SVS/MS, Ananindeua, Pará, Brasil
IIIHospital Universitário João de Barros Barreto, Universidade Federal do Pará, Belém, Pará, Brasil
IVNúcleo de Medicina Tropical, Universidade Federal do Pará, Belém, Pará, Brasil
VCasa Dia Centro de Ats em Doenças Infecciosas Adquiridas, Belém, Pará, Brasil
Correspondence
Endereço para correspondência
Dirección para correspondencia
ABSTRACT
The aim of this study was to determine the distribution of HIV-1 subtypes in patients undergoing highly active antiretroviral therapy (HAART) therapeutic failure in Amazonas and Pará, two States in northern Brazil, from 2002 to 2006. This study was performed using plasma collected from individuals with human immunodeficiency virus-1 (HIV-1) and/or acquired immunodeficiency syndrome (AIDS) who were selected from the National Genotyping Network (Rede Nacional de Genotipagem - RENAGENO). From 2002 to 2006, a total of 127 plasma samples from the States of Amazonas and Pará, in northern Brazil, were obtained from AIDS and/or HIV-positive patients and subjected to genotyping and resistance testing using the ViroSeqTM Genotyping System kit. Using the genetic information obtained from the HIV-1 protease and/or reverse transcriptase regions, HIV-1 subtype B was identified in 85% of the cases, followed by subtype F1 (4.6%) and the recombinant forms BF1 (4.6%) and CF1 (0.8%). The results of this study were similar to the results of other studies conducted in other regions of Brazil, with the exception of the detection of recombinant CF1, which was described for the first time in the Amazon Region.
Keywords: HIV-1; Subtypes; CF1 Recombinant Form; HAART Therapeutic Failure.
RESUMO
O objetivo deste estudo foi determinar a distribuição dos subtipos de HIV-1 em pacientes com falha terapêutica à highly active antiretroviral therapy (HAART) nos Estados do Amazonas e Pará, Região Norte do Brasil, entre 2002 e 2006. Este estudo foi realizado utilizando-se soro coletado de indivíduos infectados com o vírus da imunodeficiência humana tipo 1 (HIV-1), e/ou portadores da síndrome da imunodeficiência adquirida (aids), selecionados a partir do banco de dados da Rede Nacional de Genotipagem (RENAGENO). Entre 2002 e 2006, foram obtidas 127 amostras sorológicas de pacientes portadores de aids e/ou HIV-positivos dos Estados do Amazonas e Pará, as quais foram submetidas a genotipagem e teste de resistência com o kit ViroSeqTM Genotyping System. Considerando as informações genéticas obtidas das regiões da protease e/ou transcriptase reversa do HIV-1, o subtipo B foi identificado em 85% dos casos, seguido do subtipo F1 (4,6%) e as formas recombinantes BF1 (4,6%) e CF1 (0,8%). Os resultados dessa pesquisa foram semelhantes aos encontrados em estudos realizados em outras regiões do Brasil, exceto pela detecção da forma recombinante CF1, que foi descrita pela primeira vez na Região Amazônica.
Palavras-chave: HIV-1; Subtipos; Forma Recombinante CF1; Fracasso Terapêutico da HAART.
RESUMEN
El objetivo de este estudio fue de determinar la distribución de los subtipos de VIH-1 en pacientes con fallo terapéutico al highly active antiretroviral therapy (HAART) en los Estados de Amazonas y Pará, Región Norte de Brasil, entre 2002 y 2006. Este estudio se realizó utilizando suero colectado de individuos infectados con el virus de inmunodeficiencia humana tipo 1 (VIH-1), y/o portadores del síndrome de inmunodeficiencia adquirida (sida), seleccionados a partir del banco de datos de la Red Nacional de Genotipado (RENAGENO). Entre 2002 y 2006, se obtuvieron 127 muestras serológicas de pacientes portadores de sida y/o VIH-positivos de los Estados de Amazonas y Pará, las que fueron sometidas a genotipado y a prueba de resistencia con el kit ViroSeqTM Genotyping System. Considerando las informaciones genéticas obtenidas de las regiones de la proteasa y/o transcriptasa reversa del VIH-1, el subtipo B fue identificado en un 85% de los casos, seguido del subtipo F1 (4,6%) y las formas recombinantes BF1 (4,6%) y CF1 (0,8%). Los resultados de esa investigación fueron similares a los encontrados en estudios realizados en otras regiones de Brasil, excepto por la detección de la forma recombinante CF1, que fue descrita por primera vezen la Región Amazónica.
Palabras clave: VIH-1; Subtipos; Forma Recombinante CF1; Fracaso Terapéutico de la HAART.
INTRODUCTION
Since the late 1970s, patients have lived with human immunodeficiency virus (HIV), a retrovirus that causes acquired immunodeficiency syndrome (AIDS). HIV, a member of the Lentivirus genus, is classified into two types, HIV-1 and HIV-2, which differ in the molecular weights of their proteins, their accessory genes and their epidemiological dispersal patterns. Although the spread of HIV-2 is restricted, HIV-1 has spread rapidly worldwide and has become a pandemic that is responsible for thousands of deaths every year1,2.
In Brazil, from 1980 to June 2011, 608,230 cases of HIV infection were reported. According to the Ministry of Health (MS), from 1980 to 2010, 241,469 people died in Brazil due to AIDS, and in 2010 alone, 11,865 Brazilians died from AIDS. Early diagnosis and treatment were key to reducing deaths of patients infected with HIV3,4,5,6.
The course of the disease varies widely among infected individuals. The transition from acute infection to the development of AIDS is defined by a CD4+ T cell count between 200 and 350 cells/mm3 for asymptomatic individuals. This definition was adopted by the Brazilian Advisory Committee of the Ministry of Health, which also recommended an earlier initiation of antiretroviral (ARV) treatment compared with the previous regimen7, a change that aimed to prevent lymphocyte counts from reaching 200 cells/mm3, and to prevent the onset of opportunistic infections8,9.
In the course of a natural infection by HIV-1, an initially high rate of viral replication occurs, leading to the production of approximately 1010 viral particles perday and a mutation rate of approximately 3.5 x 10-5 nucleotides per replication cycle. This high mutation rate contributes to the appearance of viral quasispecies, i.e., closely related variants of the virus that are genetically distinct from each otherand that can infect the same cell9,10,11.
The HIV-1 subtypes are classified based on phylogenetic analysis and are distributed into the main (M), outlier (O) and non-M/non-O (N) groups. The most prevalent is group M, which is divided into subtypes, subtypes (A1, A2, B, C, D, F1, F2, G, H, J, K) and circulating recombinant forms (CRFs) that are hybrids between different subtypes12,13,14,15.
Most HIV-1 samples that have been tested fall into a defined set of subtypes. However, a small number of HIV-1 samples contain genomes with regions from multiple subtypes; these circulating, recombinant forms are found in geographical areas where multiple subtypes of the virus coexist. These hybrid samples are the products of recombination events that occur in HIV-1. If two different subtypes infect a single cell, a mosaic virus can be generated that includes regions from each of the two subtypes.
Equatorial Africa presents a great diversity of group N and O variants. All group M subtypes and several CRFs co-circulate in Cameroon, Equatorial Guinea, Gabon and the Democratic Republic of the Congo16. In Brazil, subtype B is the most prevalent, followed by F, C and CRFs17,18,19.
This study aimed to assess the proportional distribution of HIV-1 subtypes in individuals living in the States of Pará and Amazonas, Northern Brazil, in the period from 2002 to 2006.
MATERIALS AND METHODS
STUDY POPULATION
The material in this retrospective study, conducted from 2002 to 2006, was processed according to the following procedures. All patients presented with highly active antiretroviral therapy (HAART) therapeutic failure.
The study population consisted of 127 patients. A total of 95 patients were from Pará State, and 32 were from Amazonas State, all of whom were monitored by RENAGENO (the National Genotyping Network) physicians. The project that originated this study, registered under CEP/IEC N° 0030/07 and CAAE 0027.0.072.00007, was submitted and approved on December 19 2007 by the Ethics Committee of the Instituto Evandro Chagas (IEC).
INCLUSION CRITERIA
We used the RENAGENO network forms, which contained personal, clinical, laboratory and epidemiological information. Subsequently, this group was evaluated using the RENAGENO criteria listed below. Patients who agreed to participate in the study signed informed consent forms from the National STD/AIDS Control Program (CN DST/AIDS) of the Brazilian Ministry of Health and from the RENAGENO network. Plasma samples (1 mL) were collected from patients who met the inclusion criteria for the study being stored in low temperature freezers at the IEC until its timely use.
Patients who were selected for genotyping examination had submitted evidence of adequate adhesion to ARV medication to avoid unnecessary use of the test.
MOLECULAR ANALYSIS
The molecular analyses began with the isolation and purification of viral RNA from patient plasma, followed by cDNA synthesis and polymerase chain reaction (PCR) amplification of the HIV-1 pol fragment, which spans the entire protease gene and approximately two thirds of the reverse transcriptase (RT) gene. The resulting 1.8-kb fragments were sequenced using the BigDye Terminator Sequencing Kit v2.0 on the ABI Prism 3100 Genetic Analyzer (Applied Biosystems, USA) coupled to DNA sequence analysis software. To determine the FASTA sequence, the sequence generated by the software was compared with the reference base sequence (HXB-2) of the B subtype of HIV-1. After comparing both sequences, it was possible to identify different genetic subtypes and/or CRFs.
Genotyping was performed on all of the samples using theViroSeqTM HIV-1 Genotyping System (Celera Diagnostic, Abbott, USA)20.
All of the nucleotide sequences were analyzed using the Stanford Sequence Resistance database21 and the National STD/AIDS Program algorithm (version from February 2005) from the Brazilian Health Surveillance Department (Secretaria de Vigilância em Saúde - SVS/MS).
This identification was performed using FASTA and genotype sequence analyses with the PN/STD/AIDS Brazilian algorithm (version from February 2004) of the SVS/MS22.
RESULTS
STUDY POPULATION CHARACTERISTICS
This study included 32 (25.2%) patients from Amazonas, 19 (59.4%) of whom were male, and 95 (74.8%) patients from Pará, 75 (78.9%) of whom were male (Table 1). All of the patients received highly active antiretroviral therapy (HAART) and met the inclusion criteria for this study.
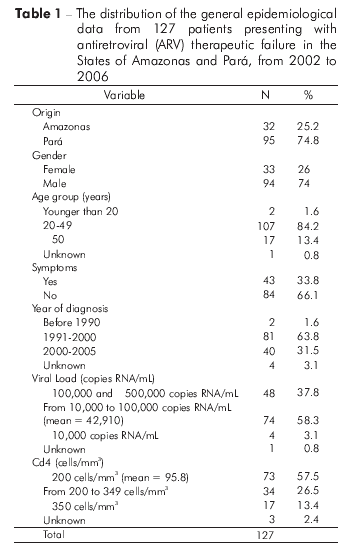
The 32 samples from the Amazonas patients were received between 2005 and 2006, and most of the samples (87.5%) belonged to asymptomatic individuals. The percentage of asymptomatic individuals was lower (58.9%) in the serum samples collected in Pará between 2002 and 2006. In both states, most patients had no clinical symptoms. In both states, the samples were collected primarily from men, and the majority of these patients (64%) were diagnosed with HIV-1 in the 1990s, 18% of whom were diagnosed in the year 1997 alone. Therefore, these patients had been living with HIV for at least ten years.
The patients' ages ranged from 19 to 72 years old (with a mean of 39 years), and most patients (107, 84.3%) were between 20 and 49 years old.
At the time of genotyping, 84 (66.1%) individuals from both States were asymptomatic, and the diagnosis of HIV-1 infection was performed between 1991 and 1999 for 63.8% of the patients (Table 1).
A total of 58.3% of the patients possessed a viral load between 10 thousand and 100 thousand RNA copies/mL (with a mean of 42,910 RNA copies/mL). The CD4+ T lymphocyte count in 57.5% of the patients at the time of inclusion in the study was below 200 cells/mm3 (with a mean of 95.8 cells/mm3) (Table1).
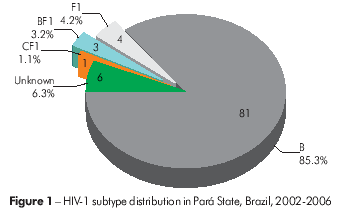
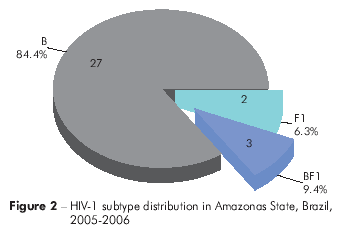
Based on the genotyping data, in Pará, 81 (85.3%) HIV-1 viral samples were classified as subtype B, four samples (4.2%) as subtype F1, three samples (3.2%) as BF1 recombinants and one sample (1.1%) asa CF1 recombinant. Additionally, six samples (6.3%) were of an unknown genotype (Figure 1). In Amazonas, 27 samples (84.4%) were classified as subtype B, two samples (6.3%) as subtype F1 and three samples (9.4%) as BF1 recombinants (Figure 2).
DISCUSSION
This study, similarly to other RENAGENO studies, investigated adult patients (with a median age of 39 years old) who were chronically infected with HIV-1 and were undergoing ARV treatment. This study included patients from the States of Amazonas and Pará, both located in northern Brazil.
All 127 patients were in virological failure; the median viral load was 67 thousand RNA copies/mL, and the median CD4+ T cell count was 171.5 cells/mm3. The viral load and the CD4+ T cell count are the primary markers used to monitor the course of AIDS in patients who are treated with ARVs4. In particular, a count of fewer than 200 CD4+ T cells/mm3 in the peripheral blood indicates the onset of an important immunodeficiency condition that is directly associated with an increase in opportunistic infections and, consequently, with the possibility of presenting a less complete therapeutic response23.
According to Morgado et al24, 131 patients in Rio de Janeiro were divided into subtypes as follows: 80.9% subtype B, 15.3% subtype F and 1% subtype D. An additional study performed a year later in this city (involving 43 samples from "blood donors") obtained similar results: 76.7% of the samples were identified as subtype B, 14% as subtype F and 9.3% as B/F or B/D recombinants18.
Brazil possesses a variety of HIV-1 subtypes, which are distributed unevenly throughout the country's geographical regions. However, in general, subtype B is the most prevalent subtype in the country25,26,27,28.
In the Brazilian Amazon, the prevalence of subtype B was greater than the prevalence of subtype F in the Cities of Belém and Macapá, Pará State. However, genetic analysis in Belém revealed the presence of env segments from subtypes B, F, D and C and the presence of pro segments from subtypes B, F, D and CRF02_AG29.
In this study, the molecular epidemiology of HIV-1 samples collected from 127 infected patients in the States of Pará and Amazonas was characterized. In addition, the proportional distribution of different subtypes in these states was estimated. Our results for the Amazon Region are similar to the results of previous studies conducted in other regions of Brazil30.
The proportion of HIV-1 subtype B infections found in this study was higher than that of subtype F1, confirming a previous report by Machado29 from the Cities of Belém and Macapá. This study differs, however, from the study by Vicente et al31 with respect to the prevalence of subtype F in Amazonas, which we observed to be nearly equal to that of subtype B. Indeed, in the present study, HIV-1 subtype B comprised 85.3% Pará and 84.4% Amazonas of HIV-1 circulating in these states, which contrasts with the results previously obtained in Amazonas State31, but another study conducted in the Amazonas revealed the high prevalence of subtype B over F and C, respectively32.
One example of HIV-1 subtype C was isolated from blood donors in Amazonas recently33.
These results support the higher prevalence of subtype B and indicate the introduction of other non-B subtypes. However, our results are consistent with those obtained by Machado29 for Pará. In contrast to subtype B, the prevalence of subtype F1 was low [Pará (4.2%), Amazonas (6.3%)], and its occurrence even included the recombinant forms B/F1 [Pará (3.2%), Amazonas (9.4%)] and CF1 (isolated only once). Notably, the detection of the latter recombinant was the first reported in Pará and the Amazon Region, and recombinant CF1 has also been described in a newly infected individual in Curitiba, Paraná State, southern Brazil34.
CONCLUSION
Based on these results, we conclude that HIV-1 subtype B was the most prevalent subtype in the samples of patients with treatment failure in the States of Amazonas and Pará, followed by subtype Fl and recombinant subtype BF1 in Pará. However, in Amazonas, recombinant subtype BF1 was more prevalent than subtype F1. In Pará, the first occurrence of recombinant CF1 in that State and the Amazon Region of Brazil was described.
ACKNOWLEDGMENTS
We thank Raimundo Macêdo dos Reis and Celina Serra de Freitas from the Instituto Evandro Chagas for their technical support.
REFERENCES
1 Barré-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May;220(4599):868-71. [Link]
2 Clavel F, Guétard D, Brun-Vézinet F, Chamaret S, Rey MA, Santos-Ferreira MO, et al. Isolation of a new human retrovirus from West African patients with AIDS. Science. 1986 Jul;233(4761):343-6. [Link]
3 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de DST, Aids e Hepatites Virais. Bol Epidemiol Aids Dst. 2012;8(1):164.
4 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Recomendações para terapia anti-retroviral em adultos infectados pelo HIV. Ministério da Saúde; 2008. [Link]
5 Vimalanand SP, Paul GF, Angela BH, Sada S, James DH, Matthew RG, et al. Cost - effectiveness of HIV sccreening in STD clinics, emergency departments, and inpatient units: a model - based analysis. PLoS ONE. 2011 May;6(5):e19936. DOI: 10.1371/journal.pone.0019936 [Link]
6 Alencastro C. De 1998 a 2010 número de novos casos subiu no país, atingindo mais os jovens gays. O Globo [Internet]. 2011 nov 29 [citado 2012 ago 11]. Disponível em: http://oglobo.globo.com/pais/de-1998-2010-numero-de-novos-casos-de-aids-subiu-no-pais-3344419.
7 Miller V, Phillips AN, Clotet B, Mocroft A, Ledergerber B, Kirk O, et al. Association of virus load, CD4 cell count, and treatment with clinical progression in human immunodeficiency virus-infected patients with very low CD4 cell counts. J Infect Dis. 2002 Jul;186(2):189-97. [Link]
8 Ledergerber B, Lundgren JD, Walker AS, Sabin C, Justice A, Reiss P, et al. Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet. 2004 Jul;364(9428):51-62. [Link]
9 Saag MS, Hahn BH, GibbonsJ, Li Y, Parks ES, ParksWP, et al. Extensive variation of human immunodeficiency virus type-1 in vivo. Nature. 1988 Aug;334:440-4. DOI:10.1038/334440a0 [Link]
10 Boyer JC, Bebenek K, Kunkel TA. Unequal human immunodeficiency virus type 1 reverse transcriptase error rates with RNA and DNA templates. Proc Natl Acad Sci USA. 1992 Aug; 89(15):6919-23. [Link]
11 Bebenek K, Abbotts J, Wilson SH, Kunkel TA. Error- prone polymerization by HIV-1 reverse transcriptase. Contribution of template-primer misalignment, miscoding, and termination probability to mutational hot spots. J Biol Chem. 1993 May;268(14):10324-34. [Link]
12 Gürtler LG, Hauser PH, Eberle J, Von Brunn A, Knapp S, Zekeng L, et al. A new subtype of human immunodeficiency virus type 1 (MVP-5180) from Cameroon. J Virol. 1994 Mar;68(3):1581-5. [Link]
13 Simon F, Mauclère P, Roques P, Loussert-Ajaka I, Müller-Trutwin MC, Saragosti S, et al. Identification of a new human immunodeficiency virus type 1 distinct from group M and group O. Nat Med. 1998 Sep;4(9):1032-7. DOI:10.1038/2017 [Link]
14 Robertson DL, Anderson JP, Bradac JA, CarrJK, Foley B, Funkhouser RK, et al. HIV-1 nomenclature proposal. Science. 2000 Apri;288(5463):55-6. [Link]
15 Vidal N, Peeters M, Mulanga-Kabeya C, Nzilambi N, Robertson D, Ilunga W, etal. Unprecedented degree of human immunodeficiency virus type 1 (HIV-1) group M genetic diversity in the Democratic Republic of Congo suggests thatthe HIV-1 pandemic originated in Central Africa. J Virol. 2000 Nov;74(22):10498-507. DOI:10.1128/JVI.74.22.10498-10507.2000. [Link]
16 Overbaugh J, Bangham CR. Selection forces and constraints on retroviral sequence variation. Science. 2001 May;292(5519):1106-9. DOI:10.1126/science.1059128 [Link]
17 Louwagie J, McCutchan FE, Peeters M, Brennan TP Sanders-Buell E, Eddy GA, et al. Phylogenetic analysis of gag genes from 70 international HIV-1 isolates provides evidence for multiple genotypes. AIDS. 1993 Jun;7(6):769-80. [Link]
18 Tanuri A, Swanson P, Devare S, Berro OJ, Savedra A, Costa LJ, et al. HIV-1 subtypes among blood donors from Rio de Janeiro, Brazil. J Acquir Immune Defic SyndrHumRetrovirol. 1999 Jan;20(1):60-6. [Link]
19 Delgado E, León-Ponte M, Villahermosa ML, Cuevas MT, Deibis L, Echeverria G, et al. Analysis of HIV type 1 protease and reverse transcriptase sequences from Venezuela for drug resistance-associated mutations and subtype classification: a UNAIDS Study. AIDS Res Hum Retroviruses. 2001;17(8):753-8. DOI:10.1089/088922201750237022. [Link]
20 Celera Diagnostic. ViroSeqTM HIV-1 Genotyping System versão 2.0: Instructions for use. ABBOTT; 2004.
21 Stanford University. HIV drug resistance database [Internet]. 2006 [cited 2006 Jul]. Available from: http://hivdb.stanford.edu/index.html.
22 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Algoritmo brasileiro do Programa Nacional de DST-Aids. Brasilia: Ministério da Saúde; 2005.
23 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Recomendações para a terapia anti-retroviral em adultos e adolescentes infectados pelo HIV Brasilia: Ministério da Saúde; 2003. [Link]
24 Morgado MG, Guimarães ML, Gripp CB, Costa CI, Neves I Jr, Veloso VG, et al. Molecular epidemiology of HIV-1 in Brazil: high prevalence of HIV-1 subtype B and identification of an HIV-1 subtype D infection in the city of Rio de Janeiro, Brazil. J Acquir Immune Defic Syndr Hum Retrovirol. 1998 Aug;18(5):488-94. [Link]
25 Morgado MG, Guimarães ML, Galvão-Castro B. HIV-1 polymorphism: a challenge forvaccine development - a review. Mem Inst Oswaldo Cruz. 2002;97(2):143-50. http://dx.doi.org/10.1590/S0074-02762002000200001 [Link ]
26 Brindeiro RM, Diaz RS, Sabino EC, Morgado MG, Pires IL, Brigido L. Brazilian network for HIV drug resistance surveillance (HIV-BResNet): a survey of chronically infected individuals. AIDS. 2003 May;17(7):1063-9. [Link]
27 Couto-Fernandez JC, Silva-de-Jesus C, Veloso VG, Rachid M, Gracie RS, Chequer-Fernandez SL, et al. Human immunodeficiency virus type1 (HIV-1) genotyping in Rio de Janeiro, Brazil: assessing subtype and drug-resistance associated mutations in HIV-1 infected individuals failing highly active antiretroviral therapy. Mem Inst Oswaldo Cruz. 2005 Feb;100(1):73-8. [Link]
28 Cavalcanti AM, Lacerda HR, Brito AM, Pereira S, Medeiros D, Oliveira S. Antiretroviral resistance in individuals presenting therapeutic failure and subtypes of the human immunodeficiency virus type 1 in the Northeast Region of Brazil. Mem Inst Oswaldo Cruz. 2007 Nov;102(7):785-92. [Link]
29 Machado LFA. Epidemiologia molecular do vírus da imunodeficiência humana tipo 1 (HIV-1) nas Cidades de Belém (Pará) e de Macapá (Amapá), Brasil. [tese]. Belém (PA): Universidade Federal do Pará, 2004.
30 Tanuri A, Caridea E, Dantas MC, Morgado MG, Mello DL, Borges S, et al. Prevalence of mutations related to HIV-1 antiretroviral resistance in Brazilian patients failing HAART. J Clin Virol. 2002 Jul;25(1):39-46. [Link]
31 Vicente AC, Otsuki K, Silva NB, Castilho MC, Barros FS, Pieniazek D, et al. The HIV epidemic in the Amazon Basin is driven by prototypic and recombinant HIV-1 subtypes B and F. J Acquir Immune Defic Syndr. 2000 Apr;23(4):327-31. [Link]
32 Jacomini DLJ. Caracterização molecular do vírus da imunodeficiência Humana tipo 1 (HIV-1) em pacientes atendidos na Fundação de Medicina Tropical do Amazonas [dissertação]. Manaus (AM): Universidade do Estado do Amazonas, Fundação de Medicina Tropical do Amazonas; 2007. [Link ]
33 Cunha LKH, Kashima S, Amarante MFC, Haddad R, Rodrigues ES, Silva KLT, et al. Distribution of human immunodeficiency virus type 1 subtypes in the State of Amazonas, Brazil, and subtype C identification. Braz J Med Biol Res. 2012 Feb;45(2):104-12. [Link]
34 Ferreira JL, Thomaz M, Rodrigues R, Harrad D, Oliveira CM, Oliveira CA, et al. Molecular characterization of newly identified HIV-1 infections in Curitiba, Brazil: preponderance of clade C among males with recent infections. Mem Inst Oswaldo Cruz. 2008 Dec;103(8):800-8. [Link]
 Correspondence / Correspondência / Correspondencia:
Correspondence / Correspondência / Correspondencia:
Olinda Macêdo
Instituto Evandro Chagas
Rodovia BR 316, km 7, s/n.
Bairro: Levilândia
CEP: 67030-000
Ananindeua-Pará-Brazil
Phone: +55(91)3214-2006
E-mail: olindamacedo@iec.pa.gov.br
Received / Recibido em / Recibido en: 28/6/2012
Accepted / Aceito em / Aceito en: 13/12/2012












 Curriculum ScienTI
Curriculum ScienTI
