Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Pan-Amazônica de Saúde
versão impressa ISSN 2176-6215versão On-line ISSN 2176-6223
Rev Pan-Amaz Saude v.5 n.1 Ananindeua mar. 2014
http://dx.doi.org/10.5123/S2176-62232014000100005
ORIGINAL ARTICLE | ARTIGO ORIGINAL | ARTÍCULO ORIGINAL
Norovirus outbreak in a cruise ship along the Brazilian coast, March 2011
Surto de norovírus em um navio cruzeiro ao longo da costa brasileira, março de 2011
Brote de norovirus en un barco de crucero a lo largo de la costa brasileña, marzo de 2011
Yvone Benchimol GabbayI; Jones Anderson Monteiro SiqueiraII; Ian Carlos Gomes de LimaI; Dielle Monteiro TeixeiraI; Glicélia Cruz AragãoIII; Luana Soares BarbagelataI; Darleise de Souza OliveiraI; Carla Gisele Ribeiro GarciaIV; Daniele de Barros GalindoV; Joana D'Arc Pereira MascarenhasI; Alexandre Costa LinharesI
ISeção de Virologia, Instituto Evandro Chagas/SVS/MS, Ananindeua, Pará, Brasil
IIPrograma de Pós-Graduação em Virologia, Instituto Evandro Chagas/SVS/MS, Ananindeua, Pará, Brasil
IIIPrograma de Pós-Graduação em Biologia Parasitária na Amazônia, Centro de Ciências Biológicas e da Saúde, Universidade do Estado do Pará, Belém, Pará, Brasil
IVVigilância em Saúde do Estado do Pará, Belém, Pará, Brasil
VCentro de Informações Estratégicas em Vigilância em Saúde, Belém, Pará, Brasil
Correspondence
Endereço para correspondência
Dirección para correspondencia
ABSTRACT
Outbreaks of gastrointestinal disease may occur on board of cruise ships due to the consumption of contaminated water or food, with a fast person-to-person transmission. Epidemiologic investigations carried out by Centers for Disease Control and Prevention in USA, have confirmed that more than 95% of gastroenteritis outbreaks in cruise ships are caused by norovirus (NoV). In March 2011 an outbreak of acute gastroenteritis (AGE) occurred on a cruise ship with 1,224 passengers and 554 crew members that sailed from Rio de Janeiro, Rio de Janeiro State (Southeast, Brazil) to Manaus, Amazonas State (North) and stopovers in Recife, Pernambuco State, and Fortaleza, Ceará State (Northeast) and Belém, Pará State (North). Epidemiological data were obtained and seven rectal swab samples were collected and tested for NoV detection and characterization by molecular techniques. A total of 53 persons (42 passengers and 11 crew members) developed AGE, 75.5% of whom were older than 60 years old. The symptoms duration was less than 48 h, most of the patients presenting vomiting (79.2%) and diarrhea (73.6%). Most of the cases varied from mild to moderate and only one patient needed parenteral rehydration. Cases of AGE were recorded in eight of 12 vessel floors, especially in the recreational areas. The seven rectal samples collected were all NoV-positive by RT-PCR and all NoV strains were genogrouped as GII by semi-nested PCR. The quantitative real-time PCR produced a 57.1% NoV positivity rate. The partial nucleotide sequencing classified five (71.4%) of these samples as GII.P4. Our findings highlight the need for continuous viral enteropathogens surveillance including cruise ships considering the increase of this kind of touristic option in Brazil.
Keywords: Norovirus; Ships; Disease Outbreaks; Epidemiological Surveillance.
RESUMO
Os surtos da doença gastrointestinal podem ocorrer em navios cruzeiros devido ao consumo de água e comida contaminadas, com rápida transmissão de uma pessoa a outra. Investigações epidemiológicas realizadas pelos Centros de Controle e Prevenção de Doenças, nos EUA, confirmaram que mais de 95% dos surtos de gastroenterite em navios são causados por norovírus (NoV). Em março de 2011, um surto de gastroenterite aguda (GEA) ocorreu em um cruzeiro com 1.224 passageiros e 554 tripulantes que partiu do Rio de Janeiro, Estado do Rio de Janeiro (Sudeste do Brasil) para Manaus, Estado do Amazonas (Norte) com paradas em Recife, Estado do Pernambuco, e Fortaleza, Estado do Ceará (Nordeste) e Belém, Estado do Pará (Norte). Dados epidemiológicos foram obtidos e sete amostras por swab retal foram coletadas e testadas para a detecção e caracterização do NoV por meio de técnicas moleculares. Um total de 53 pessoas (42 passageiros e 11 tripulantes) desenvolveram AGE, dos quais 75,5% eram maiores de 60 anos de idade. Os sintomas duraram menos de 48 h, a maioria dos pacientes apresentou vômito (79,2%) e diarreia (73,6%). A maior parte dos casos obteve variação entre leve e moderado e apenas um paciente necessitou de reidratação parental. Casos de AGE foram registrados em oito dos 12 andares do navio, principalmente nas áreas de recreação. As sete amostras de swab retal coletadas revelaram resultado positivo para NoV por meio de RT-PCR e todas as cepas foram genogrupadas como GII pelo semi-nested PCR. O PCR quantitativo em tempo real produziu uma taxa de positividade de 57,1% para NoV. O sequenciamento parcial de nucleotídeos classificou cinco (71,4%) das sete amostras como GII.P4. Os resultados destacam a necessidade de vigilância contínua dos enteropatógenos virais, incluindo navios cruzeiros, considerando o aumento deste tipo de opção turística no Brasil.
Palavras-chave: Norovirus; Navios; Surtos de Doenças; Vigilância Epidemiológica.
RESUMEN
Brotes de enfermedades gastrointestinales pueden surgir en barcos de crucero debido al consumo de agua y comida contaminadas, con una rápida transmisión de persona a persona. Investigaciones epidemiológicas realizadas por los Centros de Control y Prevención de Enfermedades, en EE. UU., confirmaron que más de 95% de los brotes de gastroenteritis en barcos son causados por norovirus (NoV). En marzo de 2011, hubo un brote de gastroenteritis aguda (GEA) en un crucero con 1.224 pasajeros y 554 tripulantes que partió de Rio de Janeiro, Estado de Rio de Janeiro (Sudeste de Brasil) para Manaus, Estado de Amazonas (Norte) con paradas en Recife, Estado de Pernambuco, y Fortaleza, Estado de Ceará (Nordeste) y Belém, Estado de Pará (Norte). Se obtuvieron datos epidemiológicos y siete muestras por hisopado rectal fueron colectadas y comprobadas para la detección y caracterización del NoV a través de técnicas moleculares. Un total de 53 personas (42 pasajeros y 11 tripulantes) desarrollaron AGE, de los cuales un 75,5% era mayor de 60 años de edad. Los síntomas duraron menos de 48 h, la mayoría de los pacientes presentó vómitos (79,2%) y diarrea (73,6%). La mayor parte de los casos tuvo una variación de leve a moderado y apenas un paciente necesitó de rehidratación parenteral. Casos de AGE se registraron en ocho de los 12 pisos del barco, principalmente en las áreas de recreación. Las siete muestras de hisopado rectal colectadas revelaron resultado positivo para NoV por medio de RT-PCR y todas las cepas fueron agrupadas como GII por hemi-Nested PCR. El PCR cuantitativo en tiempo real lanzó una tasa positiva de 57,1% para NoV. La secuenciación parcial de nucleótidos clasificó cinco (71,4%) de las siete muestras como GII. P4. Los resultados destacan la necesidad de vigilancia continua de los enteropatógenos virales, incluyendo barcos de crucero, considerando el aumento de este tipo de opción turística en Brasil.
Palabras clave: Norovirus; Navíos; Brotes de Enfermedades; Vigilancia Epidemiológica.
INTRODUCTION
Norovirus-related acute gastroenteritis (AGE) outbreaks have been described in a variety of settings, frequently in closed communities such as institutionalized persons, nursing homes, day care centers, university dormitories and cruise ships1,2.
The management of norovirus (NoV) outbreaks on cruise ships is complex since it requires rapid notification to local public health authorities by the crew members, as well as to the national agencies of the visited countries3. Gastrointestinal illness outbreaks can occur on board of cruises due to the consumption of contaminated water, ice, or food, with a fast person-to-person transmission. The risk of disease increases because of the number of days passengers spend on board4,5,6.
Epidemiologic investigations carried out by the Vessel Sanitation Program (VSP) at the Centers for Disease Control and Prevention (CDC), in USA, have confirmed that more than 95% of AGE outbreaks on cruise ships are caused by NoV Infections by this virus are difficult to control on cruise ships considering that several places are shared by passengers, including dining room and recreational areas. The fast flow of passengers and the several opportunities to introduce NoV on board may also increase the likelihood of spread that virus7.
NoV is a single-stranded RNA virus that infects all age groups, with more seriouness in young children, elderly people, and persons with chronic illness. Its infections generally courses as a mild and short-lived illness, although occasionally complications and deaths may occur8. NoV is highly contagious as it can easily propagate for foodborne and waterborne, by person-to-person contact, contaminated surfaces or fomites, and by vomit9. The minimum infective dose is less than 100 particles, and NoV is resistant to most of common control mechanisms such as the detergents used routinely. Control measures to prevent NoV outbreak comprise isolation of people with AGE symptoms, proper hand washing, personal care, reducing exposure to people affected and facility closure. The appropriate hand hygiene continues to be the most efficient way to reduce the viral load, while hand sanitizer remains an effective adjunct10,11,12,13. Considering the high infectivity of NoV and its environmental resistance, hygiene and educational measures are not sufficient to avoid repeated outbreaks by that virus14,15,16.
Despite biannual sanitation monitoring, even with hand hygiene interventions among passengers and crew members, 66 ships monitored by the CDC have experienced NoV infection outbreaks since 200317. In addition, there was an increase in registered number of NoV outbreaks on cruise ships in Europe. A total of 43 outbreaks of confirmed NoV infection occurred in 13 European-based cruise ships, from 2002 to 200618.
In 2007, the Health Protection Agency (HPA), the Association of Port Health Authorities (APHA) and the Maritime and Coastguard Agency (MCA) in the United Kingdom (UK), published guidelines for the management of NoVs infection in cruise ships. These guidelines set out the roles and responsibilities of public health agencies and port health authorities in the UK involved in the outbreaks management on cruise ships, and provide recommendations for travel companies. However, although guidance from other countries also exists, there is no international standard agreement on this subject3,19,20.
In March 2010, an outbreak of AGE was found out in Brazil, among people who had eaten meal at a cruise ship. The NoV was supposed to be the main agent responsible for this outbreak and for this reason the suspected food items implicated in this event were tested by molecular methods at the Núcleo de Doenças Entéricas of Instituto Adolfo Lutz in São Paulo, Brazil. Both genotypes GI and GII of NoV were detected distinctly in five food samples collected suggesting more than one source of contamination21.
This paper described a NoVs-related AGE outbreak aboard a cruise ship docked in Belém, Pará State, and Manaus, Amazonas State, Amazon Region of Brazil.
MATERIAL AND METHODS
EPIDEMIOLOGIC INVESTIGATION
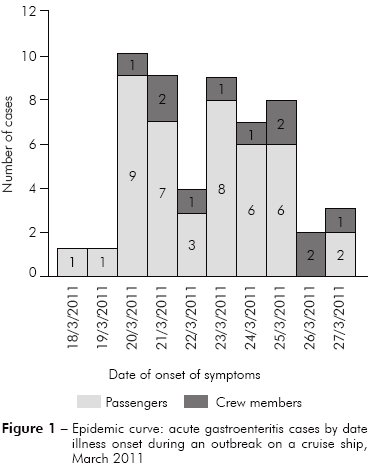
In March 2011 an outbreak of AGE was reported on a cruise ship that departed from Rio de Janeiro (Rio de Janeiro State) to Recife (Pernambuco State), Brazil, with 1,224 passengers (the majority were foreigners) and 554 crew members. The first vomiting and diarrhea case occurred in a male passenger on March 18, 2011, at the very day that the ship departed from Rio de Janeiro to Recife, but the case was only reported to the ship medical staff on March 20. A second case happened at the day after (March 19) involving a female passenger and reported on March 20. Additional new cases (n = 10) were registered in March 20, including the index case's wife. Other new cases were documented on subsequent days until March 27 (Figure 1).
When the ship arrived in Recife, Northeast of Brazil, the local health surveillance office collected water samples and fecal material of crew members and passengers with AGE. Later, the vessel departed to the Amazon Region with a first stopover in Belém, Pará State, North of Brazil. These events warned the Center for Strategic Information in Health Surveillance (Centro de Informações Estratégicas e Respostas em Vigilância em Saúde - CIEVS) and the Coordination of Food and Waterborne Diseases (Coordenação de Vigilância das Doenças de Transmissão Hídrica e Alimentar) from the Brazilian Ministry of Health, to guide local health authorities on strategies and measures to be adopted. The National Health Surveillance Agency (Agência Nacional de Vigilância Sanitária - ANVISA) and the Health Surveillance of Pará State were responsible for the investigation, with the gathering of epidemiological data and clinical samples. It was discovered that 53 people developed gastrointestinal symptoms. Later, in Manaus, new cases were reported by the Amazonia Health Service, but no epidemiological data on these further episodes was available.
The use of the samples and information obtained during this ship outbreak was authorised by the Ethics Committee in Research in Humans of the Instituto Evandro Chagas, under register CAAE:526.786.
LABORATORY ANALYSIS
Seven rectal swabs placed on Cary Blair transport medium (three from Belém and four from Manaus) were obtained by ANVISA and sent to IEC to be tested for NoVs. These samples were from patients who presented AGE symptoms at the time of collection. The samples were submitted to RNA extraction by the guanidine isothiocyanate- silica method22. A random hexamer [9 A260 units/µl, 3 mM Tris-HCl (pH 7.0), 0.2 mM EDTA - Invitrogen, Eugene, Oregon, USA] was used in the reverse transcription reaction to obtain the complementary DNA (cDNA). The cDNA amplification was done using RT-PCR with different pairs of primers. First, the pool of primers Mon 432/434 and Mon 431/43323 that detects the NoVs genogroups I and II, respectively were used and the semi nested RT-PCR was also used with the primers JV13I/JV12Y. Finally, GI/JV13I (genogroup I) and JV12Y/NoroII-R (genogroup II)24 were used in the second step. All primers described previously aim a partial region of the RNA polymerase gene. The region D (capsid gene) was also used as well as, the degenerate primers Cap A/Cap B1/Cap B2 and Cap C/Cap D1/Cap D3 for GI and GII genotyping, respectively25. The thermocycling conditions were those described by Vinjé et al25. The real time PCR or quantitative polymerase chain reaction (qPCR) involved a pair of primers COG1-F/COG1-R and the fluorescent probes G1a/G1b to detect the genogroup I, and primers COG2-F/COG2-R combine with the G2 probe for genogroup II26.
MOLECULAR CHARACTERIZATION
The amplicons were purified using the QIAquick® PCR Purification Kit and QIAquick® Gel Extraction Kit (QIAGEN, Science, Maryland, USA). The sequencing was performed by capillary electrophoresis in ABI Prism 3130XL DNA Sequencer (Applied Biosystems, Foster City, USA). The nucleotide sequences obtained were aligned and edited by the BioEdit Sequence Alignment Editor (v. 7.0.9.1) software and compared with other sequences obtained from GenBank database. The dendogram was constructed by the neighbor-joining method using the MEGA 5 software27, supported by bootstrap procedures using over 2,000 replicates. The sequences determined in this study were submitted to the GenBank under accession numbers JX987784-JX987788.
RESULTS
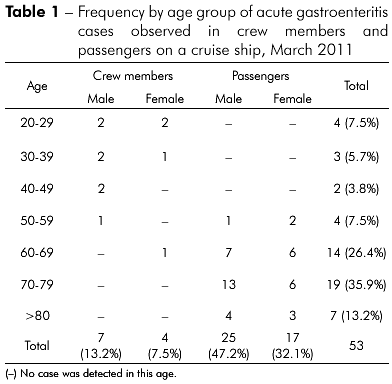
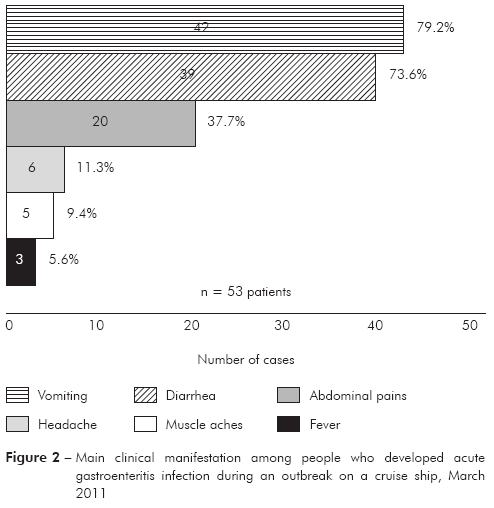
As based on data recorded on standard medical forms filled by official health personnel, 53 persons (3.4%-42/1,224 passengers and 2.0%-11/554 crew members) developed AGE and 75.5% were older than 60 years old and all below 50 years old were crew members (Table 1). Figure 1 shows the case's distribution, considering the onset of the symptoms, which in most of the cases remained less than 48 h. The main symptoms presenting by the patients were: vomiting (42/53-79.2%), diarrhea (39/53-73.6%) and abdominal cramps (20/53-37.7%) (Figure 2). In most of the cases the AGE was either mild as moderate severity and only one patient needed intravenous rehydration. Seven passengers had ten to 20 evacuations and two of them had ten to 12 vomiting episodes. In six cases both husband and wife had AGE, and the beginning of the symptoms ranged from one to four days between the cases.
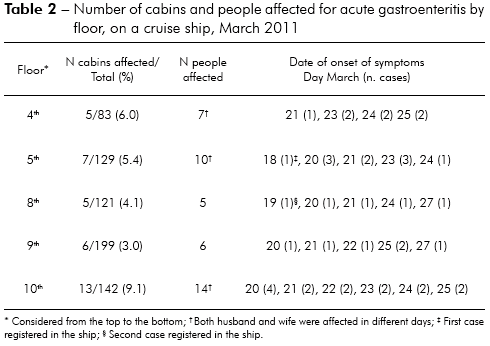
Among 12 floors of the vessel, AGE cases were recorded in eight, especially in the recreational areas. The greater number of cabins (13) with AGE cases was situated in the tenth floor where the infirmary and doctor's office were also located. Table 2 described the number of passengers and cabins with AGE cases floor by floor.
Several emergency measures were undertaken to contain the outbreak. Educational activities were passed on by radio to the passengers with the most appropriate preventive measures such as the use of alcohol gel and, subsequently hydrogen peroxide (0.5%) to clean doorknobs, computers, exercise equipments and other objects of common use. All symptomatic patients were isolated in their cabins for at least 48 h after symptoms had ceased.
The seven samples (three from Belém and four from Manaus) were NoVs positive by conventional RT-PCR and all were genogruped as GII by semi-nested PCR. Two of these samples were from crew members. The RT-PCR for the capsid region showed inconclusive results due to the presence of multiple bands. The qPCR showed positivity rate in four of the seven (57.1%) samples. The partial nucleotide sequencing of the polymerase region, classified five (71.4%) of these samples as GII.P4, two by region A and three by region B (Figure 3). The sequences obtained in the other two samples were unsuitable for analysis, due to the lack of nucleotide bases.
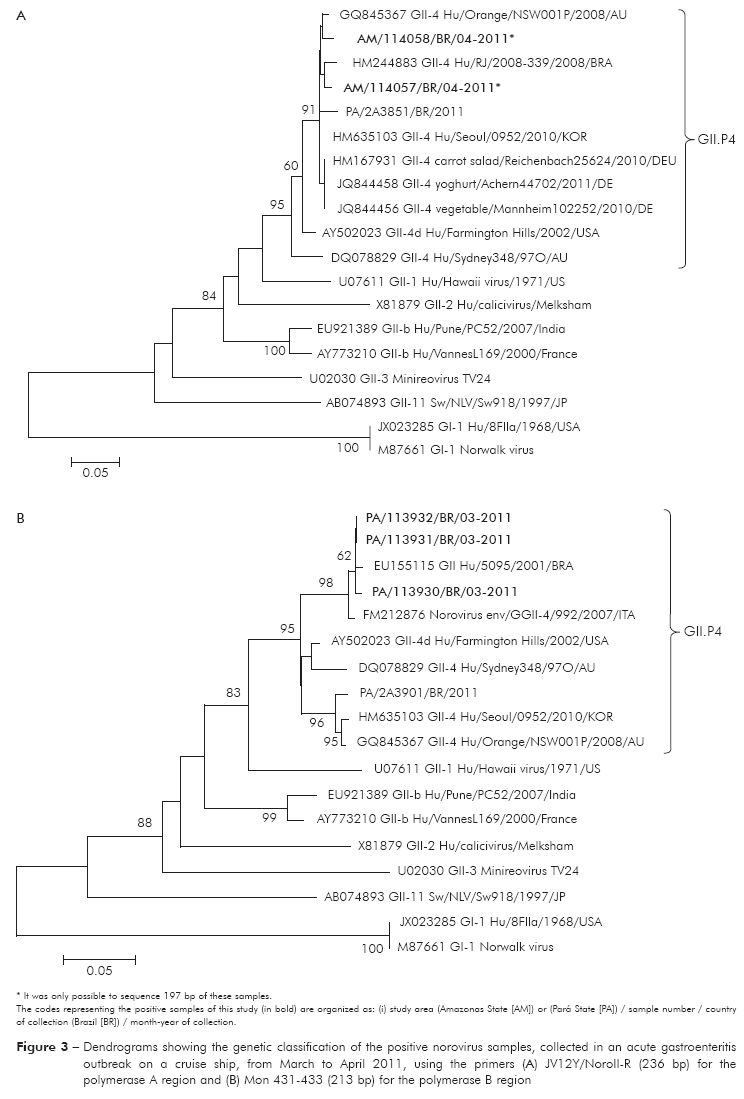
DISCUSSION
This study reports a NoV outbreak among passengers and crew members on board of a cruise ship along the Brazilian coast with stops in the cities of Belém and Manaus, Amazon Region, during March 2011. There have been a few reports on the occurrence of AGE outbreaks on cruise ships along the Brazilian coast28. In January 2009, newspapers had reported NoVs outbreak in Santos, São Paulo State, on a cruise ship that affected more than 350 ship passengers. Besides, the newspaper reports and news published on the Internet indicated that apparently the first implication of the present ship in an outbreak of AGE, occurred on March 31, 2010, when this ship docked in the port of Recife. It was registered by ANVISA and the disease affected 19 people (three crew members). Another outbreak was also reported in this same ship on late November 2011, that sailed from Valparaiso, a Chilean coastal city, to Montevideo, Uruguay. During this outbreak a total of 86 cases of gastroenteritis were recorded involving 79 passengers and seven crew members, emphasizing that an elderly woman died. However, it was not possible to conclude if this case was related to gastroenteritis, because the woman had diabetes, hypertension and heart disease. The involvement of the NoVs in those episodes were confirmed by the Laboratório de Virologia Comparada e Ambiental of Instituto Oswaldo Cruz (IOC/Fiocruz)29.
In our investigation, that outbreak apparently began in Rio de Janeiro, but it was only reported in Recife, as well as most passengers with AGE were older than 60 years. The same situation was observed in another outbreak that occurred on a cruise ship around the British Isles in 2010, where most of the affected people were in this age group3.
In our study, most of the patients developed vomiting (79.2%) and diarrhea (73.6%). The prevalence of vomiting cases was similar to an outbreak that occurred on a cruise ship in Alaska, 2004 (74%) and in another cruise ship in 2009 (75%)30,31. However, the positivity rate obtained for diarrhea in this study (73.6%) was lower than those reported in those two outbreaks (100%) and (94%)30,31.
The partial nucleotide sequencing of the NoV samples tested in this study, strongly suggested that genotype GII.P4 was responsible for the outbreak. This same genotype and genotype GI were detected in a foodborne outbreak occurred on a cruise ship in Brazil in 201021. A study conducted by Fioretti et al32, focusing on genetic diversity of NoVs in Brazil, demonstrated that genotype GII.4 is highly predominant in most of the regions. The genotype GII.P4 detected in this outbreak was similar to other GII strains that circulated in Belém, Brazil, causing AGE in children admitted to a pediatric hospital, in the same period. This same genotype was reported to be predominant (68.4%) in outbreaks that occurred in healthcare institutions for the elderly people, acute care hospitals, catering establishments, cruise ships, community events, school camps, child-related settings, and consumption of contaminated shellfish, between 2002 and 2009 in New Zealand33. In Europe, GII.4 was identified on a cruise ship in the summer of 2006 and in 43 outbreaks that occurred in 13 vessels, including new variants (GII.4 2006a and GII.4 2006b)18,34.
The NoV spread during the outbreak may be related to delaying or hiding notification of symptoms and the lack of good hygiene practices (e.g. hand washing) as described previously30,35. Nevertheless, asymptomatic viral shedding should not be ruled out. It is likely that control measures promptly adopted by the cruise ship staff were crucial to control a broader spread of the virus, since four of the 12 vessel floors had no reported cases. It is also likely that the use of alcohol gel was not the appropriated preventive measure in contamination by NoVs, considering that this product has limited effectiveness in eliminating this virus36. Also this outbreak could be related to contaminated food, as occurred in March 2010 in a cruise ship docked in São Paulo coast21.
The Brazilian Ministry of Health published in 2011 an official manual on how to handle outbreaks on cruise ships37.
The very few number of samples obtained (n = 7) is a limitation of our study. Moreover, the apparently low positivity rate achieved by qPCR and nucleotide sequencing, might possibly be due to a low viral concentration provided by rectal swabs, and also by the use of Cary Blair to storage this specimen. It is important to mention that the qPCR was used to confirm the results, but in a qualitative rather than quantitative way. Also, the epidemiological information involving the entire outbreak is not available.
CONCLUSION
Our study highlights the need for continuous surveillance for viral enteropathogens in cruise ships, with emphasis to NoV. This surveillance becomes imperative because shore excursions tend to increase in Brazil and NoV is currently gaining importance as the cause of endemic disease all over the world; in addition there is a current concern on the rapid increase in the emergence of NoV variant GII.4 -Sydney 2012 around the world, already detected in Brazil.
ACKNOWLEDGEMENTS
The authors would like to thank the crew and passengers of this Cruise Ship X for their assistance with the investigation and the healthcare professionals who gave generously their time to collect the data upon which this report is based. This investigation would not have been possible without technical, logistic, and administrative support from Túlio Fumian, Maria Silvia de Lucena, Paula Spada, Juliana Hernandez, Thaís de Carvalho and Beatriz Pedroza.
REFERENCES
1 Minooee A, Rickman LS. Infectious diseases on cruise ships. Clin Infect Dis. 1999 Oct;29(4):737-43. [Link]
2 Parashar U, Quiroz ES, Mounts AW, Monroe SS, Fankhauser RL, Ando T, et al. Norwalk-like viruses: public health consequences and outbreak management. MMWR Recomm Rep. 2001 Jun;50(RR-9):1-17. [Link]
3 Vivancos R, Keenan A, Sopwith W, Smith K, Quigley C, Mutton K, et al. Norovirus outbreak in a cruise ship sailing around the British Isles: investigation and multi-agency management of an international outbreak. J Infect. 2010 Jun;60(6):478-85. Doi:10.1016/j.jinf.2010.03.018 [Link]
4 Daniels NA, Neimann J, Karpati A, Parashar UD, Greene KD, Wells JG, et al. Traveler's diarrhea at sea: three outbreaks of waterborne enterotoxigenic Escherichia coli on Cruise Ships. J Infect Dis. 2000 Apr;181(4):1491-5. Doi:10.1086/315397 [Link]
5 Lawrence DN. Outbreaks of gastrointestinal diseases on cruise ships: lessons from three decades of progress. Curr Infect Dis Rep. 2004 Apr;6(2):115-23. Doi:10.1007/s11908-996-0007-7 [Link]
6 Rooney RM, Bartram JK, Cramer EH, Mantha S, Nichols G, Suraj R, et al. A review of outbreaks of waterborne disease associated with ships: evidence for risk management. Public Health Rep. 2004 Jul-Aug;119(4):435-42. Doi: 10.1016/j.phr.2004.05.008 [Link]
7 Centers for Disease Control and Prevention. Outbreak updates for international cruise ships: vessel sanitation program 2011 [Internet]. [cited 2011 Dec 3]. Available from: http://www.cdc.gov/nceh/vsp/surv/GIlist.htm.
8 Goodgame R. Norovirus gastroenteritis. Curr Infect Dis Rep. 2007 Mar;9(2):102-9. Doi:10.1007/s11908-007-0004-5 [Link]
9 Hall AJ, Vinjé J, Lopman B, Park GW, Yen C, Gregoricus N, et al. Updated norovirus outbreak management and disease prevention guidelines. MMWR Recomm Rep. 2011 Mar;60(RR-3):1-18. [Link]
10 Kampf G, Ostermeyer C. Efficacy of alcohol-based gels compared with simple hand wash and hygienic hand disinfection. J Hosp Infect. 2004 Apr;56 Suppl 2:S13-5. Doi:10.1016/j.jhin.2003.12.026 [Link]
11 Duizer E, Koopmans M. Efficacy of ethanol-based hand rubs. J Hosp Infect. 2005 Dec;61(4):362-3. Doi:10.1016/j.jhin.2005.06.003 [Link]
12 Fretz R, Svoboda P, Schorr D, Tanner M, Baumgartner A. Risk factors for infections with Norovirus gastrointestinal illness in Switzerland. Eur J Clin Microbiol Infect Dis. 2005 Apr;24(4):256-1. Doi:10.1007/s10096-005-1310-1 [Link]
13 Cheng FWT, Leung TF, Lai RWM, Chan PKS, Hon EKL, Ng PC. Rapid control of norovirus gastroenteritis outbreak in an acute paediatric ward. Acta Paediatr. 2006 May;95(5):581-6. Doi:10.1080/08035250500449874 [Link]
14 Centers for Disease Control and Prevention. Outbreaks of gastroenteritis associated with noroviruses on cruise ships-United States, 2002. MMWR Morb Mortal Wkly Rep. 2002 Dec;51(49):1112-5. [Link]
15 Isakbaeva ET, Widdowson MA, Beard RS, Bulens SN, Mullins J, Monroe SS, et al. Norovirus transmission on cruise ship. Emerg Infect Dis. 2005 Jan;11(1):154-8. [Link]
16 Heijne JC, Teunis P, Morroy G, Wijkmans C, Oostveen S, Duizer E, et al. Enhanced hygiene measures and norovirus transmission during an outbreak. Emerg Infect Dis. 2009 Jan;15(1):24-30. Doi:10.3201/eid1501.080299 [Link]
17 Carling PC, Bruno-Murtha LA, Griffiths JK. Cruise ship environmental hygiene and the risk of norovirus infection outbreaks: an objective assessment of 56 vessels over 3 years. Clin Infect Dis. 2009 Nov;49(9):1312-7. Doi:10.1086/606058 [Link]
18 Verhoef L, Depoortere E, Boxman I, Duizer E, van Duynhoven Y, Harris J, et al. Emergence of new norovirus variants on spring cruise ships and prediction of winter epidemics. Emerg Infect Dis. 2008 Feb;14(2):238-43. Doi:10.3201/eid1402.061567 [Link]
19 Health Protection Agency Norovirus Working Group. Guidance for the management of norovirus infection in cruise ships: report 2007 [Internet]. [cited 2011 Dec 6]. Available from: http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1206520183347.
20 Centers for Disease Control and Prevention. Vessel sanitation program operations manual: report 2005 [Internet]. [cited 2011 Dec 6]. Available from: http://www.cdc.gov/nceh/vsp/operationsmanual/opsmanual2005.pdf.
21 Morillo SG, Luchs A, Cilli A, Timenetsky MCST. Rapid detection of norovirus in naturally contaminated food: foodborne gastroenteritis outbreak on a cruise ship in Brazil, 2010. Food Environ Virol. 2012 Sep;4(3):124-9.Doi: 10.1007/s12560-012-9085-x [Link]
22 Boom R, Sol CJA, Salimans MMM, Jansen CL, Wertheim-van Dillen PME, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990 Mar;28(3):495-503. [Link]
23 Anderson AD, Garrett VD, Sobel J, Monroe SS, Fankhauser RL, Schwab KJ, et al. Multistate outbreak of Norwalk-like virus gastroenteritis associated with a common caterer. Am J Epidemiol. 2001 Dec;154(11):1013-9. Doi:10.1093/aje/154.11.1013 [Link]
24 Boxman IL, Tilburg JJHC, Te Loeke NAJM, Vennema H, Jonker K, Boer E, et al. Detection of noroviruses in shellfish in the Netherlands. Int J Food Microbiol. 2006 May;108(3):391-6.
25 Vinjé J, Hamidjaja RA, Sobsey MD. Development and application of a capsid VP1 (region D) based reverse transcription PCR assay for genotyping of genogroup I and II noroviruses. J Virol Methods. 2004 Mar;116(2):109-17. Doi:10.1016/j.ijfoodmicro.2006.01.002 [Link]
26 Kageyama T, Kojima S, Shinohara M, Uchida K, Fukushi S, Hoshino FB, et al. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J Clin Microbiol. 2003;41(4):1548-57. Doi:10.1128/JCM.41.4.1548-1557.2003 [Link]
27 Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011 Oct;28(10):2731-9. Doi:10.1093/molbev/msr121 [Link]
28 Chagas GDC, Souza JM, Pereira MM, Lobo RP, Campos AMS, Oliveira SRS, et al. Investigação de surto diarréico em navio de cruzeiro, no Estado do Rio de Janeiro. Conferência internacional em epidemiologia-vigilância epidemiológica no século 21: programa e livro de resumo; 2010 nov 29-30; São Paulo: Secretaria de Estado da Saúde de São Paulo; 2010. 171 p.
29 Agência FIOCRUZ de Notícias. Casos de diarreia em passageiros de navio foram associados aos norovírus [Internet]. [citado 2011 dez 6]. Disponível em: http://www.fiocruz.br/ccs/cgi/cgilua.exe/sys/start.htm?sid=9.
30 Chimonas MAR, Vaughan GH, Andre Z, Ames JT, Tarling GA, Beard S, et al. Passenger behaviors associated with norovirus infection on board a cruise ship, Alaska, May to June 2004. J Travel Med. 2008 May-Jun;15(3):177-83. Doi:10.1111/j.1708-8305.2008.00200.x [Link]
31 Wikswo ME, Cortes J, Hall AJ, Vaughan G, Howard C, Gregoricus N, et al. Disease transmission and passenger behaviors during a high morbidity Norovirus outbreak on a cruise ship, January 2009. Clin Infect Dis. 2011 May;52(9):1116-22. Doi:10.1093/cid/cir144 [Link]
32 Fioretti JM, Ferreira MSR, Victoria M, Vieira CB, Xavier MPTP, Leite JPG, et al. Genetic diversity of noroviruses in Brazil. Mem Inst Oswaldo Cruz. 2011 Dec;106(8):942-7. Doi:10.1590/S0074-02762011000800008 [Link]
33 Greening GE, Hewitt J, Rivera-Aban M, Croucher D. Molecular epidemiology of norovirus gastroenteritis outbreaks in New Zealand from 2002-2009. J Med Virol. 2012 Sep;84(9):1449-58. Doi:10.1002/jmv.23349 [Link]
34 Verhoef L, Boxman IL, Duizer E, Rutjes SA, Vennema H, Friesema IHM, et al. Multiple exposures during a norovirus outbreak on a river-cruise sailing through Europe, 2006. Eurosurveillance. 2008 Apr-Jun;13(4-6):1-6. [Link]
35 Neri AJ, Cramer EH, Vaughan GH, Vinjé J, Mainzer HM. Passenger behaviors during norovirus outbreaks on cruise ships. J Travel Med. 2008 May-Jun;15(3):172-6. Doi:10.1111/j.1708-8305.2008.00199.x [Link]
36 Weinstein RA, Said MA, Perl TM, Sears CL. Gastrointestinal flu: norovirus in healthcare and long-term care facilities. Clin Infect Dis. 2008 Nov;47(9):1202-8. Doi:10.1086/592299 [Link]
37 Ministério da Saúde (BR). Agência Nacional de Vigilância Sanitária: Guia sanitário para navios de cruzeiro. Brasília: Ministério da Saúde; 2011. 69 p.[Link]
 Correspondence / Correspondência/ Correspondencia:
Correspondence / Correspondência/ Correspondencia:
Yvone Benchimol Gabbay
Instituto Evandro Chagas,
Seção de Virologia Rodovia BR 316, km 7, s/no.
Bairro: Levilândia CEP: 67030-000
Ananindeua-Pará-Brasil
Phone #: +55 (91) 3214-2015 / (91) 3214-2085
E-mail: yvonegabbay@iec.pa.gov.br
Received / Recebido em / Recibido en: 10/9/2013
Accepted / Aceito em / Aceito en: 20/3/2014












 Curriculum ScienTI
Curriculum ScienTI
