Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Pan-Amazônica de Saúde
versão impressa ISSN 2176-6215versão On-line ISSN 2176-6223
Rev Pan-Amaz Saude v.5 n.1 Ananindeua mar. 2014
http://dx.doi.org/10.5123/S2176-62232014000100007
COMMUNICATION | COMUNICAÇÃO | COMUNICACIÓN
Antifungal activity of Rosmarinus officinalis Linn. essential oil against Candida albicans, Candida dubliniensis, Candida parapsilosis and Candida krusei
Atividade antifúngica de Rosmarinus officinalis Linn. óleo essencial contra Candida albicans, Candida dubliniensis, Candida parapsilosis e Candida krusei
Actividad antifúngica de Rosmarinus officinalis Linn. aceite esencial contra Candida albicans, Candida dubliniensis, Candida parapsilosis y Candida krusei
Lurdete Maria Rocha GauchI; Simone Soares PedrosaII; Renata Antunes EstevesII; Fabíola Silveira-GomesIII; Ely Simone Cajueiro GurgelIV; Alberto Cardoso ArrudaV; Silvia Helena Marques-da-SilvaVI
IPrograma de Pós-graduação em Biologia de Agentes Infecciosos e Parasitários, Instituto de Ciências Biológicas, Universidade Federal do Pará, Belém, Pará, Brasil. Faculdade de Odontologia, Instituto de Ciências da Saúde, Universidade Federal do Pará, Belém, Pará, Brasil
IIFaculdade de Odontologia, Instituto de Ciências da Saúde, Universidade Federal do Pará, Belém, Pará, Brasil
IIIPrograma de Pós-graduação em Biologia de Agentes Infecciosos e Parasitários, Instituto de Ciências Biológicas, Universidade Federal do Pará, Belém, Pará, Brasil
IVLaboratório de Botânica, Museu Paraense Emílio Goeldi, Belém, Pará, Brasil
VLaboratório de Extração, Instituto de Ciências Exatas e Naturais, Universidade Federal do Pará, Belém, Pará, Brasil
VILaboratório de Micologia, Seção de Bacteriologia e Micologia, Instituto Evandro Chagas/SVS/MS, Belém, Pará, Brasil
Correspondence
Endereço para correspondência
Dirección para correspondencia
ABSTRACT
The antifungal and anti-germ tube formation activity of Rosmarinus officinalis Linn. essential oil was tested against four Candida strains (C. albicans, C. dubliniensis, C. parapsilosis and C. krusei). Inhibition halo sizes and minimal inhibitory concentrations (MIC) were obtained using radial diffusion and micro dilution tests, respectively. The minimal fungicidal concentration (MFC) was obtained from the MIC assay. Additionally, the effect of the essential oil on germ tube formation in C. albicans and C. dubliniensis was evaluated. The MIC50 ranged from 0.5% to 2%, while the MFC ranged from 1% to 2%. We observed total inhibition of germ tube formation in C. albicans and C. dubliniensis. The R. officinalis Linn. essential oil displayed powerful inhibitory and fungicidal activity against specific Candida strains.
Keywords: Candida Species; Rosmarinus officinalis; Essential Oil.
RESUMO
A atividade de formação do tubo antifúngico e antigerme do óleo essencial de Rosmarinus officinalis Linn. foi testado contra quatro cepas de Candida (C. albicans, C. dubliniensis, C. parapsilosis e C. krusei). Halos de inibição e concentração inibitória mínima (MIC) foram obtidos utilizando os testes de difusão radial e de microdiluição, respectivamente. A concentração fungicida mínima (MFC) foi obtida por meio de ensaio da MIC. Além disso, o efeito do óleo essencial na formação do tubo germinativo de C. albicans e C. dubliniensi foi avaliado. A MIC50 variou de 0,5% a 2%, enquanto a MFC variou de 1% a 2%. Observou-se a inibição total do crescimento do tubo germinativo em C. albicans e C. dubliniensis. O óleo essencial de R. officinalis Linn. demonstrou potente atividade inibitória e fungicida contra cepas específicas de Candida.
Palavras-chave: Candida; Rosmarinus officinalis; Óleos Voláteis.
RESUMEN
La actividad de formación del tubo antifúngico y anti-germen del aceite esencial de Rosmarinus officinalis Linn. fue verificada en cuatro cepas de Candida (C. albicans, C. dubliniensis, C. parapsilosis y C. krusei). Se obtuvieron halos de inhibición y concentración inhibitoria mínima (MIC) utilizando las pruebas de difusión radial y de microdilución, respectivamente. La concentración fungicida mínima (MFC) se obtuvo a través de ensayo de la MIC. Además, se evaluó el efecto del aceite esencial en la formación del tubo germinativo de C. albicans y C. dubliniensi. La MIC50 varió de 0,5% a 2%, mientras que la MFC varió de 1% a 2%. Se observó la inhibición total del crecimiento del tubo germinativo en C. albicans y C. dubliniensis. El aceite esencial de R. officinalis Linn demostró una potente actividad inhibitoria y fungicida contra cepas específicas de Candida.
Palabras clave: Candida; Rosmarinus officinalis; Aceites Volátiles.
INTRODUCTION
The uses, effects, and pharmacological properties of medicinal plants have been widely investigated in phytotherapy1. Studies have shown that some aromatic plants and spices possess inhibitory activities against bacteria and yeast2,3. The specific anti-Candida activities of some extracts4,5,6, essential oils7,8,9 and their purified components10,11,12 are well known. Similarly, Rosmarinus officinalis Linn. extract has been demonstrated to be active against Streptococcus sanguinis (ATCC 10556), Streptococcus mutans (ATCC 25175), Streptococcus sobrinus (ATCC 27609), Lactobacillus casei (ATCC 7469)13 and Herpes Virus type-114, and it has also been shown to act as an anti-oxidative stress agent in diabetes15. Lima et al.16 reported the activity of R. officinalis Linn. essential oil against C. albicans (ATCC-76615). However, few assays have further investigated this activity in C. albicans and other Candida species. Thus, the present study examined the antifungal activity of R. officinalis Linn. essential oil against C. albicans, C. dubliniensis, C. parapsilosis and C. krusei by determining the effective inhibitory and fungicidal concentrations as well as evaluating the effect of R. officinalis Linn. essential oil on germ tube formation in related species.
MATERIALS AND METHODS
STRAINS AND GROWTH CONDITIONS
This study used C. albicans (INCQS 49175), C. dubliniensis (CBS 7987), C. parapsilosis (ATCC 29019) and C. krusei (ATCC 6258). All of the strains were grown on Sabouraud dextrose Agar (Difco Laboratories, Detroit/MI) under aerobic conditions at 37o/C for 24 h before the antifungal assays. The yeast (107 cells/mL) suspensions used in the assays were prepared in sterile phosphate-buffered saline (PBS) at pH 7.2.
OBTAINING R. officinalis LINN.
Rosmarinus officinalis Linn. was grown in the botanical garden Jacques Huber, located on the Coordination of Botany, the research campus of the Museu Paraense Emílio Goeldi (Belém, Pará State, Brazil). Plants were grown under 50% shade, potted in black polyethylene with dimensions of 20 X 25 cm, filled with black soil substrate and irrigated as needed to maintain the humidity of the substrate. The specimens were cultured during the rainy season and were not exposed to any type of chemical, such as pesticides or fertilizers. The botanical materials collected (by Gauch, L.M.R 01) were held at the growing site, and the phenophase was adult flower buds. R. officinalis Linn. was identified by Ely Simone Cajueiro Gurgel (Museu Paraense Emílio Goeldi, Pará State, Brazil) using MG 204.248.
PREPARATION OF R. officinalis LINN. ESSENTIAL OIL
The essential oil was obtained by steam distilling fresh leaves (350 g) for 240 minutes using the Clevenger system. Two milliliters of the essential oil was obtained from this process, and it was stored in a dark, cool place.
ANTIFUNGAL TESTS
The agar diffusion method was conducted as previously described17. Briefly, Petri plates containing Sabouraud dextrose agar (Difco Laboratories, Detroit/MI) were inoculated with yeast suspension using a swab. After 15 minutes, 5 mm wells were made, and 100 µ.L of essential oil was deposited into each well. We tested the susceptibility of the yeasts by radial diffusion using both pure essential oil and an emulsion of essential oil at 8% (8% essential oil, 0.8% Tween 80 in water), as described by Allegrini et al18. The plates were incubated at 37o/C for 24 h, and the inhibition halo was measured in millimeters.
The minimum inhibitory concentrations (MIC) were obtained using a microdilution test in 96-well cell culture plates as described by Prabuseenivasan et al19 using an emulsion of the essential oil (mentioned above) at concentrations ranging from 4% to 0.007% (v/v). The plates were read at A630 (TP-Reader, Thermoplate) after a 48-h incubation at 37o/C. MIC50 (defined as the minimum concentration that inhibited 50% of the isolate tested such that DO630 ≤ 0.05) was used as the endpoint of inhibition. Next, 10 µ.L from each well was inoculated on Petri plates containing Sabouraud dextrose agar (Difco Laboratories, Detroit/MI) and incubated at 37o/C for five days to determine the MFC (minimal fungicidal concentration). The MFC (defined as the lowest concentration without visible growth) was used as the endpoint for fungicidal effects.
EFFECT OF R. officinalis LINN. ESSENTIAL OIL ON GERM TUBE FORMATION BY C. ALBICANS AND C. DUBLINIENSIS
The effect of R. officinalis Linn. essential oil on germ tubes was evaluated as described by Bernardes et al20. Briefly, germ tube formation was rapidly induced in Sabouraud dextrose broth using (10%) fetal bovine serum and a solution of either 4% essential oil from R. officinalis Linn. and 0.02% Tween 80 (assay tube) or nothing (control tube), in a final volume of 10 mL (optimization of the concentration of essential oil; unpublished observations). The tube was inoculated with a C. albicans or C. dubliniensis suspension (100 µL), and the test was conducted at 37o/C for over 3 h. The total number of cells and germ tubes that formed was determined on a Neubauer chamber. The results were described as the percentage of germ tube forming cells out of the total number of cells.
STATISTICAL ANALYSIS
The statistical differences were evaluated using BioEstat version 5.3 software (ANOVA, Tukey test). P < 0.05 was considered significant.
RESULTS
Candida strains were susceptible to the R. officinalis Linn. essential oil. Using the agar-diffusion method, we observed inhibition halos ranging from 39 to 47 mm using pure essential oil. Essential oil at a final concentration of 8% had an inhibition halo ranging from 9 to 13 mm. The most susceptible strain was C. albicans, for which the MIC50 was 0.5%. C. dubliniensis and C. krusei displayed MIC50 values of 1% (75% growth inhibited). We determined that C. parapsilosis presented the highest MIC50 (2%). Interestingly, while essential oil at a concentration of 2% was able to inhibit greater than 90% of growth for this species, essential oil at a 1% concentration inhibited only 40% of growth. Thus, the MFC was 1% for C. albicans and C. krusei and 2% for C. dubliniensis and C. parapsilosis. These results are summarized in table 1.
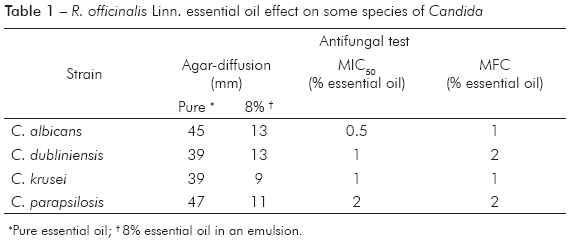
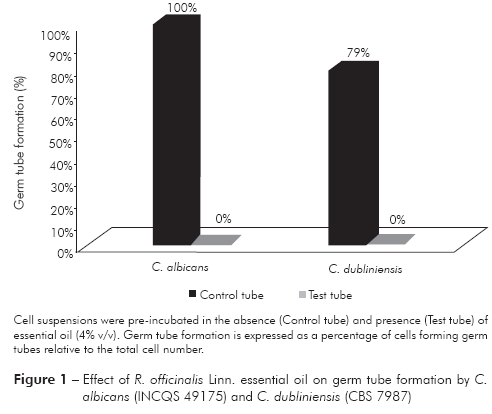
After incubation with the essential oil, germ tube formation was completely inhibited in both C. albicans and C. dubliniensis (p = 0.01) (Figure 1).
DISCUSSION
Some authors have previously studied the antibacterial activity of R. officinalis Linn.13,21,22,23, but few reports have tested the activity of medical plant extracts against strains of Candida spp. However, these studies often present conflicting results because there are no standardized techniques for evaluating their antifungal activity24 (unlike the evaluation of antifungal drugs, which has been standardized by Clinical and Laboratory Standards Institute M27-A3 methodology25). Large inhibition halos, ranging from 39 to 47 mm, were obtained from pure essential oil, indicating that it is an effective antifungal. Previous studies using an emulsion of R. officinalis Linn. essential oil at a final concentration of 8% indicated that some yeast strains are resistant (C. albicans FCF-243 and C. parapsilosis ME-2) and some are susceptible (C. albicans ATCC-76615, C. krusei FCF-281 and C. parapsilosis MD-6), with inhibition halos ranging from 10 to 13 mm16. If we had used the endpoint of susceptibility described by these authors, only C. krusei would have been classified as resistant to an 8% concentration of oil. However, the susceptibility results of these previous studies corroborate our current findings, as the inhibition halos from our 8% essential oil emulsion ranged from 9 to 13 mm, confirming the important anti-Candida activity of R. officinalis Linn. essential oil. It is difficult to establish the limits of susceptibility and resistance with the agar diffusion test for essential oils because there is no standardized methodology8,26. However, it has been suggested that 60% of all essential oils tested possess antifungal activity5.
Interesting results were obtained for C. krusei and C. parapsilosis. Using the essential oil emulsion, we observed 50% inhibition of C. krusei at 0.5% oil and of C. parapsilosis 1% oil. Identical values for the MIC50 and MFC were obtained for both species. Nascimento et al8 reported that assays testing the antifungal activity of essential oils could be inconsistent due to factors such as volatility, water solubility, and viscosity. In the present study, these factors were minimized by including Tween 80 as a surfactant, improving the homogeneity of the emulsion, and by keeping the plates in the dark to minimize the degradation of the volatile essential oil. We demonstrated that the susceptibility profiles of C. krusei and C. parapsilosis vary in this study, and we hypothesize that the R. officinalis Linn. essential oil tested in the present study contains high concentrations of active compounds such as α-Pinene and 1,8-Cineole27. Further studies are underway to determine which fractions of the R. officinalis Linn. essential oil were responsible for the observed activity and whether there is any synergy among active compounds.
Aloe vera extract has been previously shown to inhibit germ tube formation in C. albicans, and the number of cells forming germ tubes was reduced by approximately 95% using a 10% concentration of extract20. Based on concentration optimization studies (data not showed), we used 4% essential oil in the germ tube formation inhibition assay. This concentration was able to inhibit 100% of germ tube formation (p = 0.01) for both C. albicans and C. dubliniensis. These results highlight the potential of R. officinalis Linn. essential oil as an antifungal drug candidate. We acknowledge the need to determinate the active compounds that inhibit germ tube formation and their mechanisms of action. Studies to characterize the composition of the oil, with the aim of determining the concentrations of the active components, are being conducted to further reported the antifungal activity of the essential oil of R. officinalis used.
CONCLUSION
We identified concentrations of R. officinalis Linn. essential oil that are able to inhibit the growth of C. albicans, C. dubliniensis, C. krusei and C. parapsilosis. Additionally, 4% essential oil totally inhibited germ tube formation in C. albicans and C. dubliniensis. These results suggest a need to further evaluate the antifungal performance of R. officinalis Linn. essential oil in clinical fungal samples.
REFERENCES
1 Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999 Oct;12(4):564-82. [Link]
2 Cosentino S, Tuberoso CIG, Pisano B, Satta M, Mascia V, Arzedi E, et al. In-vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett Appl Microbiol. 1999 Aug;29(2):130-5. Doi: 10.1046/j.1472-765X.1999.00605.x [Link]
3 Pina-Vaz C, Rodrigues AG, Pinto E, Oliveira SC, Tavares C, Salgueiro L, et al. Antifungal activity of Thymus oils and their major compounds. J Eur Acad Dermatol Venereol. 2004 Jan;18(1):73-8. Doi:10.1111/j.1468-3083.2004.00886.x [Link]
4 Alves PM, Leite PHAS, Pereira JV, Pereira LF, Pereira MSV, Higino JS, et al. Atividade antifúngica do extrato de Psidium guajava Linn. (goiabeira) sobre leveduras do gênero Candida da cavidade oral: uma avaliação in vitro. Rev Bras Farmacogn. 2006 abr-jun;16(2):192-6. Doi:10.1590/S0102-695X2006000200010 [ Link]
5 Menezes TOA, Alves ACBA, Vieira JMS, Menezes SAF, Alves BP, Mendonça LCV. Avaliação in vitro da atividade antifúngica de óleos essenciais e extratos de plantas da região amazônica sobre cepa de Candida albicans. Rev Odontol UNESP. 2009 mai-jun;38(3):184-91. [Link]
6 Matos BM, Komiyama EY, Balducci I, Koga-Ito CY. Atividade antifúngica do extrato alcoólico de Mentha piperita sobre Candida albicans e C. tropicalis. Rev Odontol UNESP. 2009 jul-ago;38(4):244-8. [Link]
7 Castro RD, Lima EO. Atividade antifúngica dos óleos essenciais de sassafrás (Ocotea odorifera Vell.) e alecrim (Rosmarinus officinalis L.) sobre o gênero Candida. Rev Bras Plantas Med. 2011;13(2):203-8. Doi:10.1590/S1516-05722011000200012 [Link]
8 Nascimento PFC, Nascimento AC, Rodrigues CS, Antoniolli AR, Santos PO, Barbosa Júnior AM, et al. Atividade antimicrobiana dos óleos essenciais: uma abordagem multifatorial dos métodos. Rev Bras Farmacogn. 2007 jan-mar;17(1):108-13. Doi:10.1590/S0102-695X2007000100020 [Link]
9 Castro RD, Lima EO. Atividade antifúngica in vitro do óleo essencial de Eucalyptus globulus L. sobre Candida spp. Rev Odontol UNESP. 2010 mai-jun;39(3):179-84. [Link]
10 Duarte MCT, Figueira GM, Sartoratto A, Rehder VLG, Delarmelina C. Anti-Candida activity of Brazilian medicinal plants. J Ethnopharmacol. 2005 Feb;97(2):305-11. Doi:10.1016/j.jep.2004.11.016 [Link]
11 Deus RJA, Alves CN, Arruda MSP. Evaluation of the antifungal effect of oleoresin and essential oil of copaiba (Copaifera multijuga Hayne). Rev Bras Plantas Med. 2011;13(1):1-7. Doi:10.1590/S1516-05722011000100001 [Link]
12 Ramage G, Milligan S, Lappin DF, Sherry L, Sweeney P, Williams C, et al. Antifungal, cytotoxic, and immunomodulatory properties of tea tree oil and its derivative components: potential role in management of oral candidosis in cancer patients. Front Microbiol. 2012 Jun;3(Art.220):1-8. Doi:10.3389/fmicb.2012.00220 [Link]
13 Silva MSA, Silva MAR, Higino JS, Pereira MSV, Carvalho AAT. Atividade antimicrobiana e antiaderente in vitro do extrato de Rosmarinus officinalis Linn. sobre bactérias orais planctónicas. Rev Bras Farmacogn. 2008 abr-jun;18(2):236-40.Doi: 10.1590/S0102-695X2008000200017 [Link]
14 Mancini DAP, Torres RP, Pinto JR, Mancini-Filho J. Inhibition of DNA Virus: Herpes-1 (HSV-1) in cellular culture replication, through an antioxidant treatment extracted from rosemary spice. Braz J Pharm Sci. 2009 Jan-Mar;45(1):127-33. Doi:10.1590/S1984-82502009000100016 [Link]
15 Silva AMO, Andrade-Wartha ERS, Carvalho EBT, Lima A, Novoa AV, Mancini-Filho J. Efeito do extrato aquoso de alecrim (Rosmarinus officinalis L.) sobre o estresse oxidativo em ratos diabéticos. Rev Nutr PUCCAMP. 2011 jan-fev;24(1):121-30. Doi:10.1590/S1415-52732011000100012 [Link]
16 Lima IO, Oliveira RAG, Lima EO, Farias NMP, Souza EL. Atividade antifúngica de óleos essenciais sobre espécies de Candida. Rev Bras Farmacogn. 2006 abr-jun;16(2):197-201. Doi:10.1590/S0102-695X2006000200011 [ Link]
17 Ficker CE, Arnason JT, Vindas PS, Alvarez LP, Akpagana K, Gbéassor M, et al. Inhibition of human pathogenic fungi by ethnobotanically selected plant extracts. Mycoses. 2003 Feb;46(1-2):29-37. Doi:10.1046/j.1439-0507.2003.00838.x [Link]
18 Allegrini M, Siméon M, Maillos H, Boiloot A. Emulsions et applications en microbiologie. Trav Soc Pharm Montp. 1973;33:73-86.
19 Prabuseenivasan S, Jayakumar M, Ignacimuthu S. In vitro antibacterial activity of some plant essential oils. BMC Complement Altern Med. 2006;6(39):1-8. Doi:10.1186/1472-6882-6-39 [Link]
20 Bernardes I, Rodrigues MPF, Bacelli GK, Munin E, Alves LP, Costa MS. Aloe vera extract reduces both growth and germ tube formation by Candida albicans. Mycoses. 2012 May;55(3):257-61. Doi:10.1111/j.1439-0507.2011.02079.x [Link]
21 Parnham MJ, Kesselring K. Rosmarinic acid. Drugs Future. 1985 Sep;10(9):756-7.
22 Petersen M, Simmonds MSJ. Rosmarinic acid. Phytochemistry. 2003 Jan;62(2):121-5. Doi:10.1016/S0031-9422(02)00513-7 [Link]
23 Valones MAA. Avaliação da atividade antimicrobiana in vitro do dentifrício à base de extrato alcoólico de Romarinus officinalis Linn (Alecrim) sobre cepas padrão de S. mutans, S. aureus e L. casei [dissertação]. Recife (PE): Universidade Federal de Pernambuco; 2008.
24 Hood JR, Wilkinson JM, Cavanagh HMA. Evaluation of common antibacterial screening methods utilized in essential oil research. J Essent Oil Res. 2003 Nov-Dec;15(6):428-33. Doi:10.1080/10412905.2003.9698631 [Link]
25 Clinical and Laboratory Standards Institute: reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard: CLSI document M27-A3 and Supplement S3. 3 ed. CLSI, Wayne, PA, USA; 2010.
26 Oliveira RAG, Lima EO, Vieira WL, Freire KRL, Trajano VN, Lima IO, et al. Estudo da transferência de óleos essenciais sobre a atividade de alguns antibióticos usados na clínica. Rev Bras Farmacogn. 2006 jan-mar;16(1):77-82. Doi:10.1590/S0102-695X2006000100014 [Link]
27 Gomes Neto NJ, Luz IS, Tavares AG, Honorio VG, Magnani M, Souza EL. Rosmarinus officinalis L. essential oil and its majority compound 1,8-cineole at sublethal amounts induce no direct and cross protection in Staphylococcus aureus ATCC 6538. Foodborne Pathog Dis. 2012 Dec;9(12):1071-6. Doi:10.1089/fpd.2012.1258. [Link]
 Correspondence / Correspondência/ Correspondencia:
Correspondence / Correspondência/ Correspondencia:
Lurdete Maria Rocha Gauch
Instituto de Ciências da Saúde,
Faculdade de Odontologia,
Universidade Federal do Pará
Av. Augusto Corrêa, 1. Bairro: Guamá
CEP: 66075-110 Belém-Pará-Brasil
Phone#.: +55 (91) 3201-7637
E-mail: lrgauch@ufpa.br
Received / Recebido em / Recibido en: 2/8/2013
Accepted / Aceito em / Aceito en: 20/3/2014













