INTRODUCTION
Candida spp., Cryptococcus neoformans and Aspergillus spp. are opportunistic pathogens and they can cause infection in immunocompromised patients1. Candida spp. are commensal and opportunistic pathogens virtually present in all humans, these species are frequently isolated from mucosal sites where they are generally considered to be colonizing organisms. However, due to Candida spp. bloodstream infections are among the major causes of morbidity and mortality in hospitalized patients2.
Candida albicans is a fungus that can be found in many body sites such as the vagina, mouth or rectum. This fungus can also cause systemic infection and induce damage to many organs, such as heart, especially in immunocompromised patients3.
The limited efficacy of the available antifungal drugs against the fungi which cause serious mycoses, mainly in HIV-positive people, added to the rapid emergence of resistant organisms which further diminishes therapeutic capabilities, have highlighted the need of new antifungal compounds which could constitute alternatives to the existing drugs4,5. It was demonstrated that Candida spp. including C. albicans, can be resistant to some antifungals such as amphotericin B, anidulafungin, fluconazole and itraconazole6. The main clinical consequence of antifungal resistance is the treatment failures7. For that reason is important to search for new antifungals.
The medicinal plants are important sources to the development of drugs with biological activity. Many plant extracts, essential oils and their constituents isolated from plants exert biological activity in vitro and in vivo. Since plants produce a variety of compounds with antimicrobial properties, may yield possible compounds for developing new antimicrobial drugs8.
Cymbopogon winterianus Jowitt ex Bor (Poaceae) is known as "citronella" and it is cultivated in India and Brazil. This medicinal plant has various uses, such as antimycotic and acaricide activities9,10.
In other studies conducted by our team, it was demonstrated that C. winterianus essential oil was active against fungus of C. albicans and minimum inhibitory concentration (MIC) against strains of C. albicans ICB 12 and ATCC 13803 was 625 µg/mL for these two strains. In this present study, the phytochemical analysis indicated that citronellal, geraniol, citronellol and eugenol were the main constituents, with percentage of 23.59%, 18.81%, 11.74% and 10.34%, respectively11.
This study aimed to evaluate the activity of C. winterianus essential oil against C. albicans, analysing the changes in morphological forms, measurement of cellular leakage and determine if oil binds to the ergosterol.
MATERIALS AND METHODS
ESSENTIAL OIL
The essential oil of C. winterianus Jowitt ex Bor was obtained from hydrodistillation by Dr. Paulo Alves Wanderley at Universidade Federal da Paraíba (UFPB). The plant identification was performed by Dr. Rita Baltazar de Lima at Botany Laboratory of the UFPB. The code of voucher specimen is JPB 41387 and it was placed in the Herbarium Professor Lauro Pires Xavier at UFPB.
FUNGAL SAMPLE
The strains of C. albicans tested were ICB 12 and ATCC 13803, they belong to the collection of the Mycological Laboratory of the UFPB.
EFFECT OF C. winterianus ESSENTIAL OIL ON THE MICROMORPHOLOGY OF C. albicans
For observation of morphological changes, it was used the microculture technique for yeasts, using agar-rice in moist chamber11,12,13,14.
C. winterianus essential oil was added to the culture medium agar-rice, in order to obtain concentrations (MIC, MICx2, MICx4). Then, on a slide glass was deposited 1 mL of agar-rice associated with the essential oil. After solidifying culture media, the yeast was seeded in two parallel striations. The striations were covered up with a sterile coverslip and a slide glass, then were incubated in a moist chamber. After 24 h, 48 h and 72 h the preparation was examined under optical microscope. In each slide was observed the presence of structural features as pseudohyphae, blastoconidia and chlamydoconidia.
LEAKAGE EFFECT
C. albicans cultures were placed on Sabouraud dextrose agar and incubated for 24-72 h at temperature 37° C. The inoculum was adjusted according to the 0.5 McFarland standard which represents 1-5 x 106 CFU/mL by adding colonies to sterile 0.85% NaCl.
Tubes with inoculum (1-5 x 106 UFC/mL) and C. winterianus essential oil at concentrations (MIC/2, MIC, MICx2 or MICx4) were incubated from 2-24 h. Aliquots of 3 mL were removed from tubes at each period of time (2, 4 and 24 h) and centrifuged at 3,000 rpm for 10 min. It was performed the absorbance analysis at 260 nm of supernatants collected in each period of time. The absorbance generated by C. albicans treated with 1.2 N HClO4 at 100° C for 30 min was considered as 100% of leakage. The values were obtained from three independent experiments and the results were expressed as means ± standard deviation (minimum value, maximum value) of the leakage percentage4,15. In order to evaluate the results it was used the ANOVA-Dunnett's test and it was considered statistically significant (p < 0.05).
ERGOSTEROL EFFECT
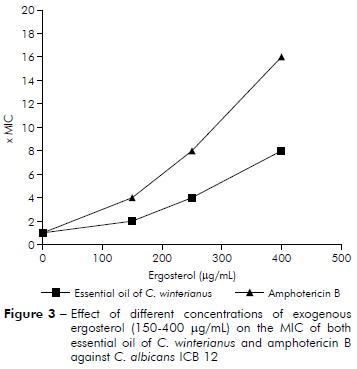
The MIC of C. winterianus essential oil against C. albicans ICB 12 was determined by the microdilution method using microplates of 96 wells in the absence and in the presence of different concentrations (150, 250 and 400 µg/mL) of ergosterol added to the medium, in different lines of the same microplate. Amphotericin B was used as a control drug. The MIC was determined after 48 h of incubation. This assay was carried out in two independent assays15,16,17.
RESULTS
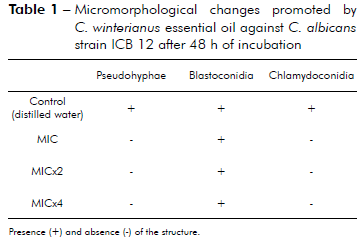
The results in table 1 show that C. winterianus essential oil at concentrations MIC (625 µg/mL), MICx2 (1,250 µg/mL) and MICx4 (2,500 µg/mL) inhibited pseudohyphae and chlamydoconidia formation in C. albicans.
Presence (+) and absence (-) of the structure.
Table 1 - Micromorphological changes promoted by C. winterianus essential oil against C. albicans strain ICB 12 after 48 h of incubation
It was evaluated if the essential oil caused damage to the fungal membrane. Measuring of intracellular materials of C. albicans at 260 nm was performed at different time intervals (2, 4 and 24 h). The figures 1 and 2 show leakage from cells 24 h after of interacting with essential oils at MIC/2, MIC, MICx2 and MICx4. The results showed that essential oil at MIC/2, MIC, MICx2 and MICx4 produced damage to the fungal membrane, evidenced by the percentage increase of cellular leakage.
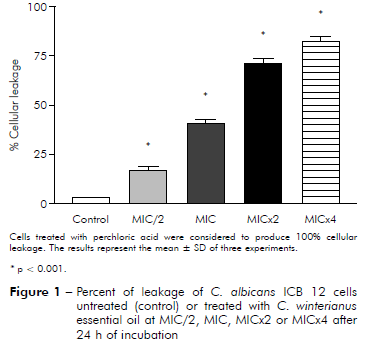
Figure 1 - Percent of leakage of C. albicans ICB 12 cells untreated (control) or treated with C. winterianus essential oil at MIC/2, MIC, MICx2 or MICx4 after 24 h of incubation
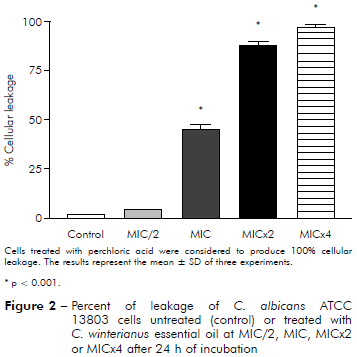
Figure 2 - Percent of leakage of C. albicans ATCC 13803 cells untreated (control) or treated with C. winterianus essential oil at MIC/2, MIC, MICx2 or MICx4 after 24 h of incubation
C. albicans ICB 12 exposed at MICx2 and MICx4 in 24 h had 71% and 82% of cellular leakage, respectively. The oil caused less leakage at MIC (about 40%) than at MICx2 and MICx4; and produced much less damage at MIC/2, approximately 16%. The cellular leakage of C. albicans ATCC 13803 after exposuring of MIC/2, MIC, MICx2 and MICx4 was about 4%, 45%, 87% and 96%, respectively. Perchloric acid was considered to produce 100% cellular leakage. Statistically, a significant difference was observed (p < 0.05) among the percentage of cellular leakage of yeast in control (without essential oil) and after exposure to the essential oil concentrations.
The results of figure 3 show that MIC of amphotericin B against C. albicans ICB 12 increased to MICx4, MICx8 and MICx16 with addition of 150, 250 and 400 µg/mL of ergosterol, respectively (MIC found to amphotericin B was 2 µg/mL11). The MIC of C. winterianus increased in the presence of ergosterol to MICx2, MICx4 and MICx8 with addition of 150, 250 and 400 µg/mL of ergosterol, respectively.
DISCUSSION
Infections caused by fungi generally present more difficult therapeutic problems than bacterial infections, which are caused by prokaryotes. The main difficulty was to find targets that do not exist in human cells. Current drug treatments are effective, but resistant strains and intrinsically resistant species are emerging fast. Natural products have diverse biological activities and may be important sources for the discovery of new antimicrobial agents18.
In this current study, it was observed the activity of C. winterianus essential oil against C. albicans, it was analysed the alterations in morphological forms, measurement of cellular leakage and determine if oil binds to the ergosterol.
Hyphae and pseudohyphae formation is related to the virulence factor expressed by C. albicans. As these structures represent a barrier to phagocytosis and allow the yeasts settlement on epithelial tissues. Morphological changes are associated with pathogenicity microorganism, and it is believed that environmental factors influence the physiological state of the commensal yeast19.
It was demonstrated that Origanum vulgare L. essential oils and Cinnamomum zeylanicum and also thymol inhibited the pseudohyphae and chlamydoconidia formation of C. albicans20,21.
In Candida spp., pseudohyphae and hyphae can grow from the yeast and the capacity of polymorphism is an important virulence factor. Both the yeast and hyphal forms are found at the site of infection and both yeast and filamentous forms have roles in infection. Several antifungal agents have morphological effects on C. albicans such as amphotericin B and azole antifungals1,22. Therefore, it is important that an antifungal inhibits the formation of hyphae, pseudohyphae and/or chlamydoconidia, because they contribute for reduce a virulence factor which help pathogenesis.
The damage to the cells can be observed by measuring the release of intracellular components to the medium from treated cells. Some cell components absorb at 260 nm, such as nucleotides that contain uracil4,15,23.
C. winterianus essential oil was used in order to cause cellular leakage against Trichophyton rubrum, suggesting that its actuation could probably involve the fungal plasma membrane24. It was reported that other essential oils compromised the structural and functional integrity of cytoplasmic membranes, which suggest that the antimicrobial mechanism occurs due to the membrane damage. Syzygium aromaticum essential oil shown fungicidal effect which resulted from extensive lesion of the cell membrane in Candida, Aspergillus and dermatophyte species25. Ocimum sanctum essential oil demonstrated antifungal activity, and the mechanism of fungicidal activity was resulted from extensive lesions of plasma membrane, it modified the structural and functional integrity of cytoplasmic membrane in Candida, leading to cell death18. The Melaleuca alternifolia essential oil exhibited antimicrobial activity against Staphylococcus aureus, and its components that damaged the cytoplasmic membrane26. Geraniol was shown to enhance the rate of potassium leakage out of whole cells and was also shown to increase bilayer permeability27.
Lipophilic compounds such as terpenes, aromatics and phenols can interact with components of cytoplasmic membrane, it may enhance the membrane permeability and loss of integrity which can be toxic to the microorganism cell28.
To determine if C. winterianus essential oil binds to the fungal membrane sterol, the MIC of this compound for C. albicans was determined with and without the addition of ergosterol. If the activity of compound was caused by binding ergosterol, it would increase the MIC of essential oil when the assay was conducted with ergosterol added to the medium15.
It was shown that Thymus vulgaris L. essential oil with ergosterol29. Some essential oil constituents such as thymol and carvacrol can also bind to ergosterol29,30.
The ergosterol has different functions in the fungus, such as bioregulator of membrane fluidity, membrane integrity, growth and membrane-bound enzymes18,31. When antifungal agents bind to the membrane ergosterol, they lead to fungal membrane disruption and causing the loss of the intracellular content15. Regarding their biological properties, essential oils are complex mixtures of numerous molecules, their biological effects are the result of a synergism of all molecules or reflect only the main molecules present at the highest levels according to the gas chromatographical analysis. It is possible that the activity of the main components is modulated by other minor molecules32.
The essential oils are composed by various compounds, the mechanism of action of essential oils may be occur due to several targets in the cell of the microorganism. The locations or mechanisms in the cell became sites of action for essential oil components that damage the cytoplasmic membrane, the membrane proteins, leakage of cell contents, causing degradation of the cell wall, coagulation of cytoplasm and depletion of the proton motive force33,34.
Considering that incidence of infections caused by fungi that has enhanced during the last decades, including the increase of resistant strains, looking for new alternatives for antifungal medication is important, and natural products, especially plants, can be the source for new drugs.