The mosquito Aedes aegypti is the main vector of Dengue virus in the Americas1 and Ae. albopictus was described as a potential vector2. In the latter species, vertical transmission of this arbovirus has been described in specimens of the mosquito collected in the field3. Since there is no safe and effective vaccine against the virus4,5, dengue prevention rely on insect control. Such actions have also become more important due to the recent spread of Chikungunya virus and Zika virus in Brazil6,7. The main strategies of integrated control of Ae. aegypti aim to eliminate (i) immature forms in breeding sites through entomological surveys and (ii) adult forms through the use of insecticides8. A simple action of covering common breeding sites (CBS) as water tank and metal drum had the effect of diminishing the number of adult female Ae. aegypti, in long-term temporal scale9. However, failure to completely suppress vector population and disease has been observed in spite of such measures. Many factors might be contributing to this failure such as fast and disorganized urbanization, insecticide resistance of insect vector, non-synchronized top-down/bottom-up control strategies10, or "epidemic-dependence" on planning and resource implementation11.
As reported, the number of dengue cases is on the rise in many places, especially in tropical and subtropical regions12. Though in Rondônia State a recent tendency of decline was observed, with a 67% reduction in 2014 in relation to 201313, the number of dengue cases has been on the rise since the first record of the virus in 1997. Virus serotype in this outbreak was not determined but in subsequent years, between 2001 and 2003, the serotype 1 was found predominant in Porto Velho14. Serotype 3 was detected in the period of 2004-2006 in the Municipalities of Ariquemes, Jaru, Ouro Preto do Oeste, Cacoal, Colorado do Oeste, Vilhena and Porto Velho15. In the year of 2009, entomological surveys were carried out due to the high number of cases reported16. A substantial number of immature mosquito forms was observed in cesspits, a place not officially listed as Ae. aegypti breeding site. Cesspits in the region are rudimentarily constructed and with loose gaskets, which freely allow insect passage. Septic tanks have been pointed as highly productive breeding places for Ae. aegypti17,18,19,20. Differently from septic tanks, however, cesspits have no outlet pipe. Therefore, waste water accumulated inside its chamber might turn cesspits even more productive. Taking into account that (i) there are no technical orientation by the National Dengue Control Program on cesspits and septic tanks during entomological surveys21,22 and that in (ii) Northern Brazil less than 10% of households are connected to public sewage systems23, such breeding places could be responsible for maintaining Ae. aegypti population density high, even with continuous and regular vector control campaigns. Hence, the present study aimed to (i) evaluate cesspits as potential and efficient breeding sites for Ae. aegypti by comparing measurements of infestation (presence/absence of larvae/pupae) in cesspit and CBS and (ii) measure some physicochemical parameters and evaluate suitability of conditions for insect oviposition and procreation in water samples from cesspits.
We conducted a transversal descriptive survey in urban areas of two Municipalities of the Rondônia State, Espigão do Oeste (6.4 inhabitants per km2; 11°31'44.9"S; 61°00'49.7"W) and Jaru (17.7 inhabitants per km2; 11°40'53.3"S; 61°10'37.6"W), Brazilian Western Amazon (Figure 1), in November and December of 2009. We randomly selected residential and commercial buildings located near the addresses of reported dengue cases in central and peripheral limits of the cities. Addresses were provided by the Municipal Health Secretary. Outdoor and indoor of households in Espigão do Oeste and Jaru (N = 8 and N = 11, respectively) were inspected by technician and researcher teams of the Research Institute of Tropical Diseases (Instituto de Pesquisas em Patologias Tropicais -IPEPATRO) and Centre for Research in Tropical Medicine (Centro de Pesquisa em Medicina Tropical - CEPEM). The area surrounding the household (peridomiciliar) up to 15 m was considered "outdoors". Water tank, plastic and metal drum, tires, pitchers, and solid waste were considered conventional CBS24 and water samples at these sites were collected according to official guidelines25. Water samples in cesspits were collected three to five times at different points of the liquid surface up to 1 L final volume, with the aid of a halved polyethylene terephthalate (PET) bottle attached to an aluminum pole. Immature mosquito forms present in the liquid sample were collected with a plastic pipette, transferred to a 2 L plastic bottle and recorded appropriately (location, date, time, etc.) (Figure 2). The immature mosquito forms were then sent to the insectary of the Laboratory of Entomology at IPEPATRO/Fiocruz and reared in water containing white bowls to adult stage for subsequent identification using taxonomic keys26. Averaged numbers of adult forms were calculated by dividing the number of mosquitoes obtained in laboratory by the total number of containers (total number of surveyed sites, positive and negative), to minimize the effect of unequal sample size in the comparisons among localities and breeding place categories. A chi-square test with Yates's correction factor was performed to compare the proportion of samples positive and negative for Ae. aegypti, in cesspits and CBS. Chi-square was based on the significance level of a = 0.05 for the comparisons and computed using the web-based software OpenEpi v. 3.0127. Water samples were collected from the cesspit and sent to the governmental laboratory of Water and Sewerage Company of Rondônia (Companhia de Aguas e Esgotos de Rondônia - CAERD), located at Espigão do Oeste Municipality. The following parameters were measured by CAERD certified technician: pH, color (Hazen Units - HU) and turbidity (Nephelometric Turbidity Units - NTU) and compared to standard values for water supplied to households by the public water system, as recommended by the Brazilian Health Ministry25. The parameters measured of water from cesspits were compared to those of drinking water and served to evaluate the range of favorable conditions for insect oviposition and procreation.
Figure 2 - Water sampling of cesspits in the Municipalities of Espigão do Oeste and Jaru, Rondônia State, Brazil
A grand total of 2,288 adult mosquitoes were obtained from the 6,556 immature forms (larvae and pupae stages) collected in cesspits (Cess: N = 19) and conventional breeding sites (CBS: N = 11) at the Municipalities of Espigão do Oeste and Jaru. Ninety one percent or 6,001 larvae were collected in Cess and the remainder 9% or 555, in CBS. The collection in Espigão do Oeste resulted in 2,042 immature forms, being 81.3% (1,661) from eight Cess and 18.7% (381) from seven CBS. In Jaru, 4,514 immature forms were collected of which 96.1% (4,340) from 11 Cess and 3.9% (174) from four CBS (Table 1).

Figure 3 - Average number of reared adults of Ae. aegypti, Cx. quinquefasciatus, and Oc. fluviatilis per Cess (N = 19) and CBS (N = 11), in Espigão do Oeste and Jaru, Rondônia State, Brazil
Three species of mosquitoes were identified using taxonomic keys on adults obtained from the collected immature forms: Ochlerotatus fluviatilis, Culex quinquefasciatus and Ae. aegypti (Table 1, Figure 3). The latter was represented by 49.0% of the total number of adult specimens obtained from the immature forms (Table 1). Almost the same percentage was observed for Cx. quinquefasciatus or 50.4% while Oc. fluviatilis had a minor frequency of 0.6%. Interestingly, Oc. fluviatilis, which is (i) used in studies of experimental avian malaria transmission in laboratory conditions28; (ii) hypothetical, but not confirmed, vector of sylvatic yellow fever; and (iii) vector of minor importance in the transmission of canine dirofilariasis26, had no previous record of its occurrence in Rondônia State.
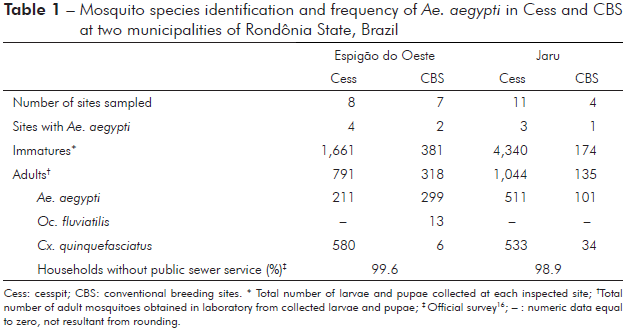
Table 1 - Mosquito species identification and frequency of Ae. aegypti in Cess and CBS at two municipalities of Rondônia State, Brazil
The relative number of adult mosquito specimens obtained from immature forms differed in respect to the mosquito species for each breeding site category. The average number of adult Ae. aegypti specimens obtained from larvae collected in Cess was similar to that from CBS, while Cx. quinquefasciatus adult specimens originated from Cess was 16 times higher than those from CBS (Figure 3).
It was observed that the rate of site infestation by Ae. aegypti is similar in both Cess and CBS (χ2 = 0.018; d.f. = 1; p > 0.05) (Table 1). However, different localities might show different rates of infestation. For instance, while in Jam the rate of infestation was almost the same in both Cess and CBS, i.e., three Ae. aegypti positive Cess from a total of 11 Cess (27%) and one positive CBS from a total of four CBS (25%) surveyed, in Espigão do Oeste the rate of infestation was 1.7 times higher for Cess (50%) than for CBS (29%) (Table 1). However, such results must be carefully interpreted since samplings were small at both localities.
Female mosquito oviposition has been correlated with turbidity29 and pH of water30. While females of Cx. quinquefasciatus have apparently preference for water with high turbidity29, those of Ae. aegypti have been long perceived to prefer clean water31. Such perception is reflected in government guidance for monitoring Ae. aegypti. Cesspits are not considered due to their obvious turbidity. Measured physicochemical properties of cesspit water samples ranged from 9.8 to 135.0 NTUs, the pH from 5.3 to 8.8, and the colour from 20.4 to 1,142.4 HUs (Table 2). For comparison purpose, the standard values of water for human consumption25 are also listed. The measured parameters of water from cesspits compared to parameters of acceptable standards for drinking water thus indicate a wider range of favorable conditions for Ae. aegypti procreation and warrant changes in the official monitoring guidance for Ae. aegypti in Brazil.

Table 2 - Turbidity, pH and color of water samples collected from cesspits in two municipalities of Rondônia State, Brazil
The difficulties experienced by some countries in integrated insect vectors control could be a combination of the opportunistic behaviour of Ae. aegypti and the less than optimal man-made structures of waste water disposal (mainly dictated by economic and social conditions), creating unexpectedly new ideal conditions for its proliferation. Septic tanks are referred in the World Health Organization guidelines for dengue control32 as liquid waste storage containers that can eventually serve as procreation environment for insect vector species. In the present study, we observed indeed that cesspits are water-holding structures equally or better suited for the breeding of this mosquito species than CBS. Of the 19 cesspits inspected in urban areas of Rondônia State, seven were positive for this species. The implication of these results is that, even with a small sampling, as performed in this study, the relevance of cesspits as breeding sites for important disease vectors is evidenced. Few studies have highlighted these findings in Brazil33,34. Furthermore, the relative productivity of cesspits compared to other water containers requires further studies with aggregated spatiotemporal parameters.
Deficiencies in public water supply for human consumption have resulted in private construction of improvised containers for water storage that are not always adequately protected10,35. Human occupation of wild areas in the tropics, often with inadequate housing structures and inefficient sewage and waste disposal systems, creates favourable conditions for an unbridled raise in Ae. aegypti density10,35. In the Amazon Region, the great majority of households have no public sewage service (Table 1). Cesspits are the main alternative for domestic waste water disposal both in rural and urban areas. However, poor construction and inadequate maintenance of cesspits, causing openings and crackings in its structure (Figure 2F), might create additional favourable conditions for mosquito procreation, contributing to increased risk of dengue outbreaks, and perhaps explaining the challenges of vector control encountered in the region. In addition, our data showed that higher levels of impurities (particulate and dissolved material) affecting water turbidity and colour as well as a wide range of pH in cesspit water, did not dissuade the mosquito from laying eggs (Table 2). During the entomological surveys, we noticed adult mosquitoes coming out of cesspit chamber, being able to collect ten to 15 adult specimens per cesspit, many of them promptly identified as Ae. aegypti (males and females). We observed winged forms in all monitored cesspits (N = 27). To ascertain that these adult mosquitoes were directly ovipositing at those sites, ovitraps were installed nearby and inside cesspits. After 24 h, the ovitraps at the different locations presented eggs, which, after taken to the laboratory and reared to adult stage, were identified in high proportion as of Ae. aegypti (results not shown).
Infestation of cesspits by Ae. aegypti is supported by results of previous studies reporting large numbers of immature and adult forms of this species in septic tanks, which are essentially improved cesspits18,19,30, independent of season and rainfall20. Septic tanks were highlighted as the main breeding site in South-Eastern Nigeria for insect's vectors of diseases, including Ae. aegypti18. In Puerto Rico, during an outbreak of dengue DEN-2, density of vector adults was restored to similar levels of pre-intervention after five weeks post-intervention, leading to the discovery of septic tanks as subterranean focus of infestation19. Adult mosquito presence in septic tanks was positively associated with wall cracking, unprotected openings and pH of septic water30.
Ae. aegypti tolerance to diverse conditions and changes in its oviposition behaviour have been reported in many previous studies. For instance, it was demonstrated that larvae of some strains of Ae. aegypti from Africa and Central America, were very tolerant to synthetic sewage36. Upflow anaerobic sludge blanket (UASB) reactor effluents as well as effluents of anaerobic filter could constitute favourable insect breeding places, based on experimental evidences of Ae. aegypti oviposition preferences37. In Malaysia, Ae. aegypti eggs and Ae. albopictus immatures were found in one toilet flush tank and in a bowl of water supply to birds38. Natural and sylvatic breeding places have been reported as outcome of spill events of Ae. aegypti populations39. In our study, the measured parameters of water from cesspits compared to parameters of acceptable standards for drinking water indicated a wider range of favorable conditions for Ae. aegypti procreation and warrant changes in the official monitoring guidance for Ae. aegypti in Brazil.
Due to climate changes caused by increasing human activities40 and the procreation adaptability shown by Ae. aegypti, new oviposition environments in conditions of natural disaster is very likely. Flood due to heavy rainfall being ever more frequent40, potentially provides new and more numerous breeding sites for this mosquito species. Prevalence of dengue has been correlated with rainfall flood in Thailand41, for instance. Such climate events cannot be ignored in the context of control of the mosquito. Moreover, the present studied region is characterized by periodic rainfall and occasional flooding. Considering that cesspits are the main infrastructure for waste water treatment in many parts of Northern Brazil23, further studies are necessary to address whether the procreation of this vector species in cesspits/septic tanks is seasonally affected by the water table, taking into account the characteristic seasonal wet and dry periods in the region.
In spite of all evidences there is no recommendation to survey septic tanks/cesspits in the Brazilian Dengue Program. Public policies should effectively include cesspit/septic tank inspection/management as a regional/local priority. Our observations of Ae. aegypti presence in a wider range of water turbidity, add in the support of such recommendations, emphasizing as well the need for more attention by governmental agencies on building better structured public sanitation, especially in developing countries situated in tropical and subtropical regions, and where dengue and other vector transmitted diseases are recurring problems.
ACKNOWLEDGMENTS
We thank the Endemic Disease Control Agents (ACE) responsible for the entomological and epidemiological surveillance and the Municipal Health Secretaries of the surveyed cities in Rondônia State. We also thank the logistic supports from CEPEM and IPEPATRO, Porto Velho, Rondônia State, Brazil.














