Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Pan-Amazônica de Saúde
versão impressa ISSN 2176-6215versão On-line ISSN 2176-6223
Rev Pan-Amaz Saude v.7 n.3 Ananindeua set. 2016
http://dx.doi.org/10.5123/s2176-62232016000300002
ORIGINAL ARTICLE
Application of the biological indices Biological Monitoring Working Party and Average Score per Taxon to assess the water quality of Ouricuri river in the Municipality of Capanema, Pará State, Brazil*
1 Laboratório de Biologia Molecular e Ambiental, Instituto Federal de Educação, Ciência e Tecnologia do Pará, Bragança, Pará, Brasil
Environmental problems related to water quality in continental ecosystems have conducted studies aimed at the protection and conservation of these environments. The current study aimed to evaluate the water quality in five points of Ouricuri river, located inside and outside of the urban area of the Municipality of Capanema, Pará State, Brazil, using the biological indices of Biological Monitoring Working Party and Average Score per Taxon. The result of the biological indices identified a pattern of water quality that was presented as doubtful (P1), critical (P2, P3 and P4) and very critical (P5), polluted water in moderate and severe form. The values of taxonomic richness tend to decrease downstream from point P2, with increased levels of turbidity, organic matter and total phosphorus and these last two are measured at substrate level. In the five sampled points, 1,039 individuals were identified which belong to 14 families with the main groups identified as Chironomidae, Oligochaeta and Thiaridae. The change in macroinvertebrate community composition along the Ouricuri river appears as a result of the combination of human factors associated with it, such as: loss of riparian forest, margin erosion, substrate change, domestic wastewater flows and proliferation of macrophytes.
Keywords: Macroinvertebrates; Bioindicators; Potability; Conservation
INTRODUCTION
Many of the changes observed in aquatic environments have resulted in large part from the expansion of agriculture and, therefore, of anthropic actions and are currently causing great concern regarding the availability and quality of water bodies1. Rivers and streams are ecosystems that have been highly impacted by population growth, the discharge of high quantities of industrial and domestic effluents, construction of dams, destruction of habitats, and introduction of exotic species2,3. These anthropic events result in imbalances in continental freshwater ecosystems and affect trophic interactions and biodiversity4. These effects result from increased nutrient and pollutant input originating from agricultural, industrial, and domestic sources5, which are the primary causes determining river quality and biodiversity decline. For this reason, much effort has been dedicated to detecting, quantifying, and mitigating these effects over the last few decades.
Natural lotic environments can be characterized by the presence, quantity, and composition of organic and inorganic agents and the diversity of their aquatic communities, and these habitats are subject to structural and seasonal variation due to internal and external factors6. The primary function of water resource monitoring is to evaluate whether certain organisms are present or absent in some regions of the lotic system. The quantity and diversity of species sensitive to frequent disturbances in these environments7, i.e., the balance between different communities in the ecosystem, are evaluated. The composition of aquatic organisms, especially invertebrates, reflects the changes in aquatic systems8 and is affected not only by factors specifically related to the water conditions but also by the dynamics between biotic and abiotic factors (biotope) of the ecosystem9.
Benthic macroinvertebrates are aquatic animals at least 0.25 mm in length that inhabit the sediment and can colonize different types of substrate, such as branches, leaves, stones, and aquatic macrophytes, during all or part of their life cycles10,11. Their distribution and the specific communities that they form in aquatic environments are affected by the substrate composition, food availability12, and the physicochemical characteristics of water13. Because the integrity of benthic macroinvertebrate communities is closely related to the presence of pollutants in the habitat, benthic macroinvertebrates can be effectively used in environmental impact studies to evaluate continental ecosystems14,15. Therefore, these animals can be used as bioindicators of water quality, allowing for evaluating the ecological effects of different water pollution sources16. They can be used in environmental quality studies due to characteristics such as their varying abundances in different aquatic ecosystems (lentic and lotic), the extent of their locomotive ability, and their presence or absence in samples before and after environmental impact events17,18,19,20.
Changes in the natural composition of benthic macroinvertebrate populations, regarding spatial dynamics, have been used as efficient biological tools in water pollution monitoring studies21. These studies gained full relevance when they described the impacts on aquatic trophic chains22. One study analyzed macroinvertebrate diversity at different points in the River Thames in the United Kingdom for one year using the Biological Monitoring Working Party (BMWP), average score per taxon (ASPT), and Shannon-Wiener diversity index to evaluate the impacts of water quality factors and physical changes to the habitats of these organisms23. The results indicated that less altered sampling sites harbored greater diversity of organisms and that water quality was the primary limiting factor of biodiversity. In Brazil, the BMWP index has been used in studies performed in the Doce River basin in the state of Minas Gerais24, in an Atlantic forest fragment also in Minas Gerais25 and in the Sinos River in the state of Rio Grande do Sul26, indicating that water quality biomonitoring studies in Brazil are still scarce and geographically restricted.
Many studies of continental aquatic environments have been performed in temperate and tropical climates using benthic macroinvertebrates as water quality indicators27,28,29,30, but few studies have been conducted in the Amazon region. This lack is the cause of great concern since many of the cities in this region are closely associated with water bodies (e.g., for transport, recreation, and food). A ten-year study of bauxite waste discharge into Lake Batata in the municipality of Oriximiná, state of Pará, reported a decrease in the density of benthic macroinvertebrates in specific locations in the lake when compared to Lake Mussurá located in the same municipality, but that was not anthropized, which raised questions related to substrate particle size and its correlation to higher or lower biodiversity29. A three-year study on physicochemical and biological conditions at five locations in the Mindu Stream in the city of Manaus in the state of Amazonas identified an apparent decrease in macroinvertebrate diversity in areas under anthropic pressure that was driven by the deforestation of riparian forests, illegal land occupation, and consequently high domestic waste disposal31. The same study also observed greater macroinvertebrate diversity in areas with less urbanization despite decreased riparian forests in those areas. A study of the water conditions at 12 points along the Maroaga River, located in Presidente Figueiredo, in the state of Amazonas classified the river as class I of the BMWP index, which means it is "clean or not significantly altered"32.
The present study aimed to evaluate the water quality conditions in a stretch of the Ouricuri River located in the municipality of Capanema in the northeastern state of Pará, using the BMWP and ASPT indices.
MATERIALS AND METHODS
Samples were taken from a stretch of the Ouricuri River where it runs along the urban perimeter of the municipality of Capanema, located approximately 165 km from Belém, the state capital of Pará (Figure 1). Five sampling sites were selected along the river: P1 (01°11'30.2"S, 47°09'36.1"W), located upriver from the city of Capanema, P2 (01°11'16.7"S, 47°10'16.0"W), P3 (01°11'22.1"S, 47°10'47.4"W), and P4 (01°11'23.8"S, 47°11'18.4"W) within the city grid, and P5 (01°11'22.8"S, 47°11'32.7"W) downriver from the city (Figure 2).
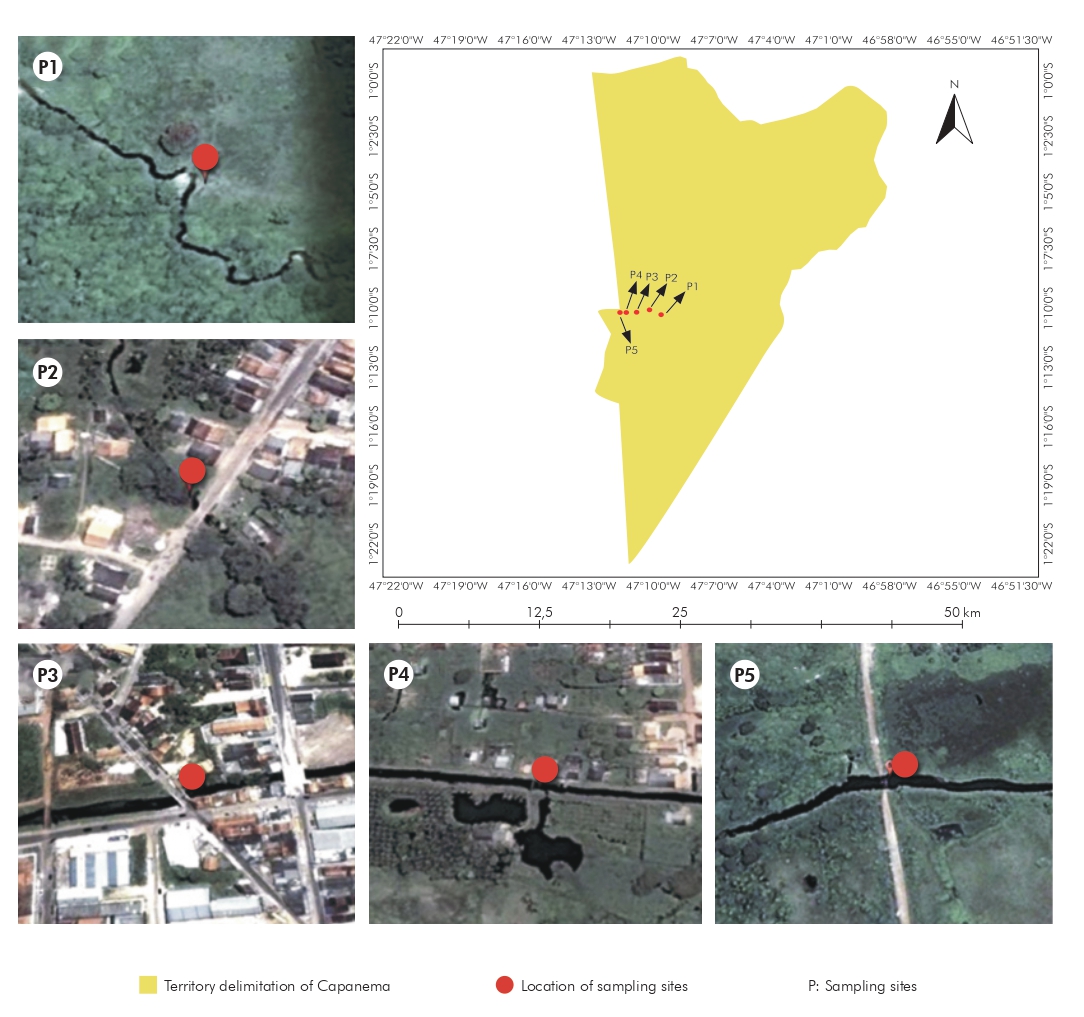
Datum: WGS84; Geographic Coordinate System Latitude/Longitude; Images: Landsat/DigitalGlobe/Google Earth Software; Map production date: February/2014.
Figure 1 - Location of benthic substrate sampling sites along the Ouricuri River in the municipality of Capanema, Pará, Brazil

P: Sampling sites.
Figure 2 - Substrate sampling sites along the Ouricuri River in Capanema, Pará, Brazil
Two substrate samples were taken at each sampling site during the drought season (October 2013). The samples were placed in 2-liter containers, labeled and transported to the laboratory, washed in running water, and filtered through a 300-μm mesh sieve, and the benthic macrofauna was preserved in 70% alcohol for subsequent analysis. According to the State of Rio de Janeiro Aquatic Macroinvertebrate Identification Manual (Manual de Identificação de Macroinvertebrados Aquáticos do Estado do Rio de Janeiro), sediment macrozoobenthos were isolated using a stereoscopic microscope, quantified, and identified to the family level33. Intact and deep profile sediment samples were obtained using a polyvinyl polychloride tubular core sampler 7.5 cm in diameter and 80 cm long.
Physicochemical parameters of the water, such as temperature (°C), dissolved oxygen (mg/L), turbidity (NTU), total phosphorus (P), and organic matter (g/Kg), were evaluated at all sampling sites. Temperature, dissolved oxygen, and turbidity were evaluated using 1-liter water samples. Total phosphorus and organic matter samples (500 g) were sent to the Brazilian Agricultural Research Corporation (Empresa Brasileira de Pesquisa Agropecuária - Embrapa), Western Amazon.
Benthic macrofauna data were analyzed using the BMWP, ASPT, and Shannon-Wiener diversity index. The BMWP index classifies the degree of resilience of benthic macroinvertebrate families using a scale from 1 to 10. High BMWP scores were attributed to highly sensitive benthic taxa and low scores to benthic taxa with a high tolerance to organic pollution, enabling their use as a tool to diagnose water body contamination by organic material34. The score for a given sampling site was obtained by adding the individual scores for all families present and the total score corresponded to a water quality class varying from good to very critical (Table 1).
Table 1 - Biological Monitoring Working Party (BMWP) classes, scores, categories, and interpretations from a water quality analysis of the Ouricuri River, Capanema, Pará, Brazil
| Class | BMWP score | Category | Interpretation |
|---|---|---|---|
| I | >150 | Good | Clean water |
| 101−150 | Clean or not significantly altered | ||
| II | 61−100 | Acceptable | Clean but slightly impaired |
| III | 36−60 | Questionable | Moderately impaired |
| IV | 15−35 | Critical | Polluted or impaired |
| V | <15 | Very critical | Heavily pollut |
The ASPT index was calculated by dividing the BMWP score by the number of families identified at a given sampling site. High ASPT scores indicate good water quality and represent a relatively high number of taxa present35 (Table 2).
Table 2 - Reference average score per taxon (ASPT) scores and interpretations used in water quality analysis for the Ouricuri River, Capanema, Pará, Brazil
| ASPT score | Interpretation |
|---|---|
| >6 | Clean water |
| 5−6 | Questionable water quality |
| 4−5 | Probable moderate pollution |
| <4 | Probable severe pollution |
Biodiversity was evaluated by calculating the Shannon-Wiener index (H') [H'=-∑(pi)(log2pi)], which supplies information on the stability of the benthic community36, using BioEstat v5.0 software. The Shannon-Wiener index evaluates species abundance in a sample considering both evenness and richness of the species present. As pollution increases, the whole benthic community is exposed to intense stress, which consequently destabilizes it. The most sensitive organisms disappear, whereas the most tolerant, due to the absence of competition for food and space, spread rapidly throughout the aquatic system resulting in decreased biodiversity and thus a decreased H'.
RESULTS
The temperature did not vary significantly between sampling sites varying within approximately 1 °C.
However, there was significant variation in dissolved oxygen, turbidity, total phosphorus, and organic matter among the five sampling sites. As the water body approximated the urban area, dissolved oxygen decreased and other parameters increased (Figure 3).
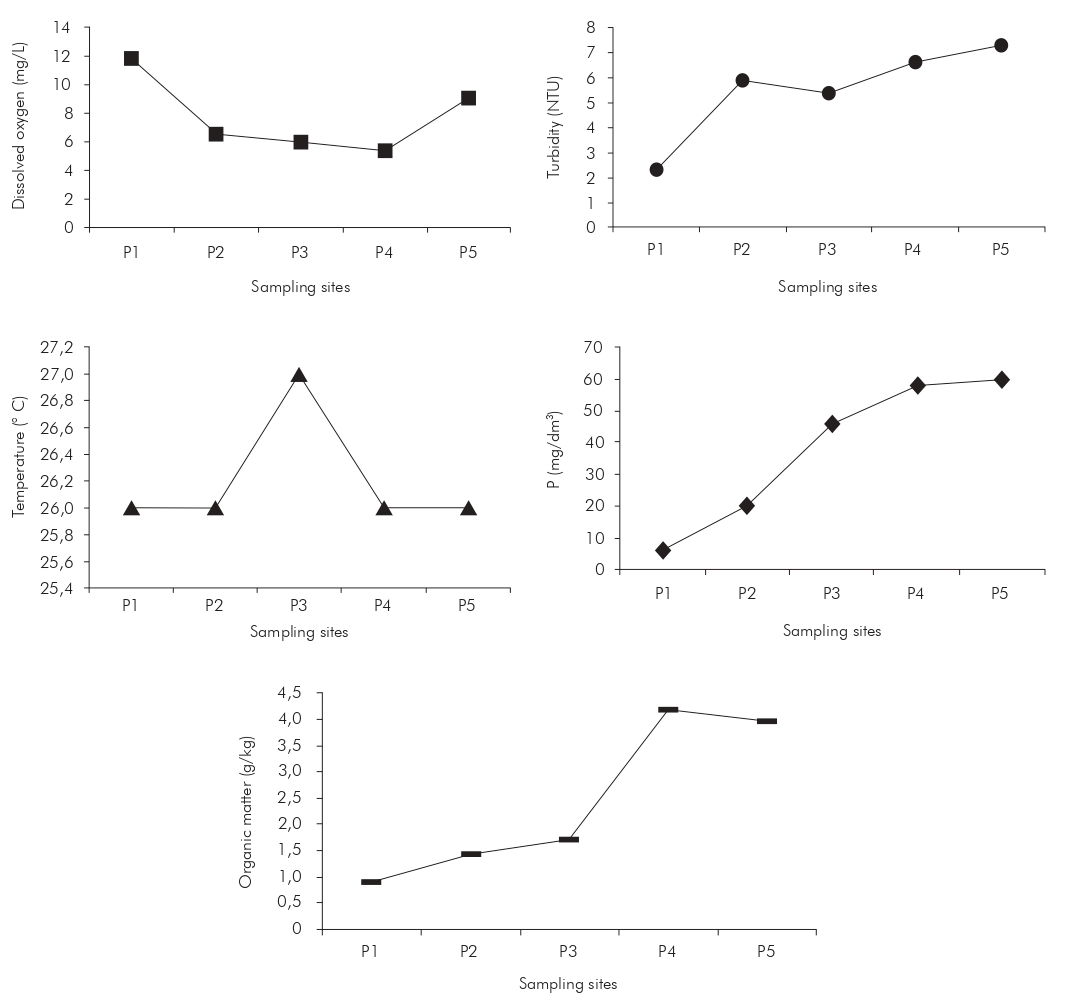
Figure 3 - Variation of physicochemical parameters in the Ouricuri River, in Capanema, Pará, Brazil, across five sampling sites
A total of 1039 individuals belonging to 14 families were collected and identified and constituted the benthic macrofauna at the five sampling sites visited in the Ouricuri River (Table 3). Following identification, the BMWP score was determined for each taxon. Of the 14 invertebrate families identified, 13 (92.8%) were found at P1 and P2, and only five (35.7%) were found at P3, P4, and P5. These results indicate an apparent decrease in biodiversity with increasing proximity to the city center. Similarly, the BMWP scores also decreased along the river (P1: 36; P2: 32; P3: 16; P4: 19; P5: 13).
Table 3 - Benthic macroinvertebrate community data from the Ouricuri River in the municipality of Capanema, Pará, Brazil
| Taxa | Tolerance index | Number of individuals per sampling site | ||||
|---|---|---|---|---|---|---|
| BMWP | P1 | P2 | P3 | P4 | P5 | |
| Diptera | ||||||
| Chironomidae | 2 | 30 | 166 | 7 | 24 | - |
| Ceratopogonidae | 4 | 1 | - | 7 | - | 2 |
| Dixidae | 4 | 1 | - | - | 2 | - |
| Culicidae | 2 | - | 1 | - | - | - |
| Physidae | 3 | - | 1 | - | - | - |
| Psychodidae | 4 | - | 2 | - | - | - |
| Trichoptera | ||||||
| Philopotamidae | 8 | 2 | - | - | - | - |
| Annelida | ||||||
| Oligochaeta | 1 | 4 | 2 | 103 | 6 | - |
| Hirudinea | 3 | - | - | 27 | 4 | - |
| Turbelaria | ||||||
| Planariidae | 5 | 4 | 4 | - | - | - |
| Gastropoda | ||||||
| Thiaridae | 6 | 83 | 319 | 24 | 158 | 12 |
| Ancylidae | 6 | 1 | 1 | - | - | - |
| Ampullariidae | 3 | - | 28 | - | - | 1 |
| Planorbidae | 3 | - | 2 | - | - | - |
| Taxa richness | 8 | 10 | 5 | 5 | 3 | |
| Total density | 126 | 536 | 168 | 194 | 15 | |
BMWP: Biological Monitoring Working Party score; P1-P5: the five sampling sites; -: Numeric data equal to zero, not resulting from rounding.
Tolerant species were more abundant at P2, P3, and P4, and the presence of pollution-sensitive organisms and indicators of low pollution levels was only observed at P1 (Table 3). The BMWP scores classified the water quality in P1 as questionable, in P2, P3, and P4 as critical, and in P5 as very critical (Table 4). The ASPT scores varied from 3.1 to 4.3 and characterized the five sampling sites as impacted environments varying from moderate (P1 and P5) to severe (P2, P3, and P4) (Table 5). Similar to the determination by the BMWP score, the environmental quality of P1 was identified as being the least anthropized.
Table 4 - Biological Monitoring Working Party (BMWP) and average score per taxon (ASPT) scores for five sampling sites in the Ouricuri River in the municipality of Capanema, Pará, Brazil
| Sampling site | Class | Color | BMWP | Quality | ASPT | Quality |
|---|---|---|---|---|---|---|
| P1 | IV | 36 | Questionable | 4,3 | Probable moderate pollution | |
| P2 | V | 32 | Critical | 3,5 | Probable severe pollution | |
| P3 | V | 16 | Critical | 3,2 | Probable severe pollution | |
| P4 | V | 19 | Critical | 3,1 | Probable severe pollution | |
| P5 | VI | 13 | Very critical | 4,3 | Probable moderate pollution |
Table 5 - Shannon-Wiener diversity index values for five sampling sites (P1-P5) in the Ouricuri River in the municipality of Capanema, Pará, Brazil
| P1 | P2 | P3 | P4 | P5 | |
|---|---|---|---|---|---|
| Sample size | 126 | 526 | 168 | 194 | 15 |
| Number of categories | 8 | 10 | 5 | 5 | 3 |
| Shannon-Wiener (H') | 0,4415 | 0,4168 | 0,4936 | 0,2868 | 0,2726 |
| Maximum diversity (H'max) | 0,9031 | 1,0000 | 0,6990 | 0,6990 | 0,4771 |
| Homogeneity (E; H'/H'max) | 0,4889 | 0,4168 | 0,7062 | 0,4103 | 0,5714 |
| Heterogeneity | 0,5111 | 0,5832 | 0,2938 | 0,5897 | 0,4286 |
Shannon-Wiener index values varied from 0.27 to 0.49, with the highest for P1, P2, and P3 (Table 5). This index is based on the proportional abundance of a species in a sample and accounts for species evenness (equitability) and richness. Homogeneity (E; Pielou's equitability index) was calculated as the ratio between diversity observed and maximum diversity ranging from 0 to 1, where 1 indicates a situation in which all species in a given sample were equally abundant. The site with the highest equitability value was P3.
DISCUSSION
Although P1 was distant from the urban area, it exhibited similar taxonomic richness to P3 and P4, which were located within the urban area and strongly impacted. This result may be due to a combination of external and internal factors, namely the decrease in the riparian forest followed by shoreline erosion and low substrate organic matter content limiting the number of taxa present. A study conducted in Lake Batata in the municipality of Oriximiná reported a decrease in macroinvertebrate diversity in a water body exhibiting a high quantity of clay-dominated sediment originating from bauxite extraction, which altered the local sediment that was originally sandy37. The increased turbidity downriver from P2 may be related to the decreased diversity observed in P3, P4, and P5.
A previous study evaluated the water and bottom sediment quality of a lake located in the municipality of Castelo in the state of Espírito Santo and showed that the instance of substrate phosphorus content greater than 40 mg/dm3 was high and could be related to eutrophication38. The presence of a considerable quantity of macrophytes observed at P5 may be related to high substrate phosphorus contents, which would directly or indirectly aid their proliferation. Phosphorus is the main eutrophication agent in continental aquatic environments, and the decrease or disappearance of some specialist species and their replacement with more tolerant generalist groups is commonly observed in impacted aquatic environments39.
The higher biodiversity observed at P2 was likely related to its less anthropized conditions, driven by higher organic matter and phosphorus levels at P3 and the remaining downriver sites. These increased values promoted increased metabolic activity in decomposers with a consequent decrease in dissolved oxygen and a reduction of the least tolerant taxa. This was confirmed by the presence of the Oligochaeta species, a bioindicator of anthropized environments, observed in higher quantities in P3.
The biological indices estimated for the sampling sites classified the Ouricuri River as questionable, critical, or very critical and moderately polluted to severely polluted. These biological index scores were lower than those observed in previous studies of continental Brazilian water bodies24,30 in which a reserved area was compared to an urbanized area in the state of Minas Gerais and benthic invertebrate diversity BMWP scores ranged from 103 to 186. The change in the composition of the aquatic macroinvertebrate fauna in the Ouricuri River directly resulted from factors such as hydrological and substrate composition changes and organic material and sediment input. Specifically, a clear increase in organic material and sediment input, accompanied by a decrease in dissolved oxygen, was observed at P2.
The Shannon-Wiener index values for the five sampling sites were equal to or lower than those observed in a study of a stream in the micro basin of the Cambará River in the municipality of Cruz Alta in the state of Rio Grande do Sul40. Based on the BMWP scores, the stream was characterized as having questionable water quality (H' = 0.46). Therefore, it was suggested that the five sampling sites analyzed had low diversity, and P4 and P5 were the most worrisome due to their low scores.
In the present study, indicator taxa of low water quality, such as Chironomidae, Oligochaeta, Planorbidae, and Planariidae, were present at sites considered altered and impacted. These benthic taxa are known to be highly resistant to anthropogenic changes, such as domestic or industrial waste discharges or the absence of riparian forests41. Dipterans belonging to the family Chironomidae were the most frequent taxa overall and were potentially responsible for a large part of the quantitative changes observed in the macrofaunal community. This is in accordance with the results of previous impact studies in which organic material discharge has been associated with urban expansion42,43,44,45. The family Chironomidae is described as the most abundant organism in aquatic environments subjected to anthropic disturbances, more frequent in areas directly affected by water quality changes due to the presence of organic pollutants originating from domestic waste discharge46,47.
ACKNOWLEDGMENTS
The authors wish to thank the Instituto Federal de Educação, Ciência e Tecnologia do Pará, campus Bragança, for the use of the Laboratory of Molecular and Environmental Biology as well as the Embrapa.
REFERENCES
1 Callisto M, Moretti M, Goulart MDC. Macroinvertebrados bentônicos como ferramenta para avaliar a saúde de riachos. Rev Bras Recur Hidr. 2001 jan-mar;6(1):71-82.[Link] [ Links ]
2 Moya N, Tomanova S, Oberdorff T. Initial development of a multi-metric index based on aquatic macroinvertebrates to assess streams condition in the Upper Isiboro-Sécure Basin, Bolivian Amazon. Hydrobiologia. 2007 Sep;589(1):107-16. Doi: 10.1007/s10750-007-0725-3[Link] [ Links ]
3 Smith RF, Lamp WO. Comparison of insect communities between adjacent headwater and main-stem streams in urban and rural watersheds. J North Am Benthol Soc. 2008 Mar;27(1):161-75. Doi: 10.1899/07-071.1[Link] [ Links ]
4 Vandewalle MF, Bello MP, Berg T, Bolger S, Doledec F, Dubs CK, et al. Functional traits as indicators of biodiversity response to land use changes across ecosystems and organisms. Biodivers Conserv. 2010 Sep;19(10):2921-47. Doi: 10.1007/s10531-010-9798-9[Link] [ Links ]
5 Meybeck M. Global analysis of river systems: from Earth system controls to Anthropocene syndromes. Philos Trans R Soc Lond B Biol Sci. 2003 Dec;358(1440):1935-55. Doi: 10.1098/rstb.2003.1379[Link] [ Links ]
6 Meybeck M, Helmer R. Introduction to water quality. In: Chapman D, editor. Water quality assessments: a guide to the use of biota, sediments and water in environmental monitoring. London: Chapman and Hall; 1992. p. 1-17. [ Links ]
7 Buss DF, Baptista DF, Nessimian JL. Bases conceituais para a aplicação de biomonitoramento em programas de avaliação da qualidade da água de rios. Cad Saude Publica. 2003 mar-abr;19(2):465-73. Doi: 10.1590/S0102-311X2003000200013[Link] [ Links ]
8 Piedras SRN, Bager A, Morais PRR, Isoldi LA, Ferreira OGL, Heemann C. Macroinvertebrados bentônicos como indicadores de qualidade de água na barragem Santa Bárbara, Pelotas, RS, Brasil. Cienc Rural. 2006 mar-abr;36(2):494-500. Doi: 10.1590/S0103-84782006000200020[Link] [ Links ]
9 Magalhães APFP. As comunidades de macro-invertebrados num sistema hidroelétrico do norte de Portugal [tese]. Porto (PT): Universidade de Porto, Faculdade de Ciências; 1989. 357 p. [ Links ]
10 Callisto M. Macroinvertebrados bentônicos. In: Bozelli RL, Esteves FA, Roland F, editores. Lago Batata: impacto e recuperação de um ecossistema amazônico. Rio de Janeiro: Sociedade Brasileira de Limnologia/UFRJ; 2000. p. 141-51. [ Links ]
11 Péres GR. Guia para el estúdio de los macroinvertebrados acuáticos del Departamento de Antioquia. Bogotá: Fen Colômbia y Colciência; 1988. [ Links ]
12 Tikkanen P, Huhta A, Muotka T. Determinants of substrate selection in lotic mayfly larvae: is cryptic coloration importante? Arch Hydrobiol. 2000;148(1):45-57. [ Links ]
13 Ward JV. Reverine landscapes: biodiversity patterns, disturbance regimes, and aquatic conservation. Biol Conserv. 1998 Mar;83(3):269-78. Doi: 10.1016/S0006-3207(97)00083-9[Link] [ Links ]
14 Li L, Zheng B, Liu L. Biomonitoring and bioindicators used for river ecosystems: definitions, approaches and trends. Proc Environm Sci. 2010;2:1510-24. Doi: 10.1016/j.proenv.2010.10.164[Link] [ Links ]
15 Qadir A, Malik RN. Assessment of an index of biological integrity (IBI) to quantify the quality of two tributaries of river Chenab, Sialkot, Pakistan. Hydrobiologia. 2009 Mar;621(1):127-53. Doi: 10.1007/s10750-008-9637-0[Link] [ Links ]
16 Belmejo L, Martos HL. Utilização de Xiphophorus helleri como bioindicador de poluição hídrica de derivados de petróleo em condições tropicais. Rev Eletr Biol. 2008;1(2):1-17.[Link] [ Links ]
17 Dornfeld CB. Utilização de análise limnológicas, bioensaios de toxicidade e macroinvertebrados bentônicos para o diagnóstico ambiental do reservatório de Salto Grande (Americana, SP) [dissertação]. São Carlos (SP): Universidade Federal de São Carlos; 2002.[Link] [ Links ]
18 Resh VH, Rosemberg DM. The ecology of aquatic insects. New York: Praeger Publishers; 1993.[Link] [ Links ]
19 Brandimart AL, Shimizu GY, Anaya M, Kuhlmann ML. Amostragem de invertebrados bentônicos. In: Bicudo CE, Bicudo DC, organizadores. Amostragem em limnologia. São Carlos: Rima; 2004. p. 213-30. [ Links ]
20 Modde T, Drewes HG. Comparison of biotic index values for invertebrate collections from natural and artificial substrates. Freshwater Biol. 1990 Apr;23(2):171-80. Doi: 10.1111/j.1365-2427.1990.tb00262.x[Link] [ Links ]
21 Sadin L, Johnson RK. The statistical power of selected indicator metrics using macroinvertebrates for assessing acidification and eutrophication of running waters. Hydrobiologia. 2000 Apr;422:233-43. Doi: 10.1023/A:1017082619481[Link] [ Links ]
22 Callisto M, Gonçalves Jr JF, Moreno P. Invertebrados aquáticos como bioindicadores. In: Navegando o Rio das Velhas das Minas aos Gerais. Belo Horizonte: UFMG; 2004. p. 1-12. [ Links ]
23 Beavan L, Sadler J, Pinder C. The invertebrate fauna of a physically modified urban river. Hydrobiologia. 2001 Feb;445(1):97-108. Doi: 10.1023/A:1017584105641[Link] [ Links ]
24 Cota L, Goulart M, Moreno P, Callisto M. Rapid assessment of river water quality using an adapted BMWP index: a practical tool to evaluate ecosystem health.Verh Internat Verein Limnol. 2002 Dec;28:1-4.[Link] [ Links ]
25 Oliveira A, Callisto M. Benthic macroinvertebrates as bioindicators of water quality in an Atlantic forest fragment. Iheringia Ser Zool. 2010 Dec;100(4):291-300.[Link] [ Links ]
26 Carvalho B, Strieder MN, Maltchik L, Stenert C. Are the streams of the Sinos River basin of good water quality? Aquatic macroinvertebrates may answer the question. Braz J Biol. 2010 Nov;70(4 Suppl):1207-15. Doi: 10.1590/S1519-69842010000600010[Link] [ Links ]
27 Lenat DR. Agriculture and stream water quality: a biological evaluation of erosion control practices. Environm Management. 1984 Jul;8(4):333-44. Doi: 10.1007/BF01868032[Link] [ Links ]
28 Ducan WFA, Brusven MA, Bjornn TC. Energy-flow response models for evaluation of altered riparian vegetation in three southeast Alaskan streams. Water Res. 1989 Aug;23(8):965-74. Doi: 10.1016/0043-1354(89)90169-3[Link] [ Links ]
29 Callisto MFP, Esteves FA. Macroinvertebrados bentônicos em dois lagos amazônicos: Lago Batata (um ecossistema impactado pelo rejeito de bauxita) e Lago Mussuri (Brasil). Acta Limnol Bras. 1996;8:137-47.[Link] [ Links ]
30 Junqueira VM, Campos SCM. Adaptation of BMWP method for water quality evaluation to rio das Velhas watershed (Minas Gerais, Brasil). Acta Limnol Bras. 1998;10(2):125-35.[Link] [ Links ]
31 Cleto-Filho SEN, Walker I. Efeitos da ocupação urbana sobre a macrofauna de invertebrados aquáticos de um igarapé da cidade de Manaus/AM - Amazônia Central. Acta Amaz. 2001 mar;31(1):69-89. Doi: 10.1590/1809-43922001311089[Link] [ Links ]
32 Uherek CB, Gouveia FBP. Biological monitoring using macroinvertebrates as bioindicators of water quality of Maroaga Stream in the Maroaga Cave System, Presidente Figueiredo, Amazon, Brazil. Int J Ecol. 2014;(ID:308149):1-7. Doi: 10.1155/2014/308149[Link] [ Links ]
33 Mugnai R, Nessimian JL, Babtista DF. Manual de identificação de macroinvertebrados aquáticos do Estado do Rio de Janeiro. Rio de Janeiro: Technical Books; 2010. [ Links ]
34 Baptista DF, Buss DF, Egler M, Giovanelli A, Silveira MP, Nessimian JL. A multimetric index based on benthic macroinvertebrates for evaluation of Atlantic Forest streams at Rio de Janeiro State, Brasil. Hydrobiologia. 2007 Jan;575(1):83. Doi: 10.1007/s10750-006-0286-x[Link] [ Links ]
35 Armitage PD, Moss D, Wright JF, Furse MT. The performance of a new biological water quality score system based on macroinvertebrates over a wide range of unpolluted running-water sites. Water Res.1983;17(3):333-47. Doi: 10.1016/0043-1354(83)90188-4[Link] [ Links ]
36 Ayres M, Ayres Junior M, Ayres DL, Santos AA. BioEstat 5.0: aplicações estatísticas nas áreas das ciências bio-médicas. Belém: MCT; IDSM; CNPq; 2007. [ Links ]
37 Esteves FA, Bozelli RL, Roland F. Lago Batata: um laboratório de limnologia tropical. Cienc Hoje. 1990;11(64):26-33. [ Links ]
38 Amaral AA, Pires SC, Ferrari JF. Qualidade da água e do sedimento de fundo de alguns córregos do município de Castelo, Estado do Espírito Santo. Rev Agroambient. 2014 mai-ago;8(2):194-203. Doi: 10.18227/1982-8470ragro.v8i2.1548[Link] [ Links ]
39 Esteves FA. Fundamentos de limnologia. 3. ed. Rio de Janeiro: Interciência; 2011. [ Links ]
40 Copatti CE, Schirmer FG, Machado JVV. Diversidade de macroinvertebrados bentônicos na avaliação da qualidade ambiental de uma microbacia do sul do Brasil. Rev Perspect. 2010 mar;34(125):79-91.[Link] [ Links ]
41 Barbosa FAR, Sousa EMM, Vieira F, Renault GPCP, Rocha LA, Maia-Barbosa PM, et al. Impactos antrópicos e biodiversidade aquática. In: Paula JA, coordenador. Biodiversidade, população e economia: uma região de Mata Atlântica. Belo Horizonte: UFMG /Cedeplar; 1997. p. 345-454.[Link] [ Links ]
42 Guereschi RM. Macroinvertebrados bentônicos em córregos da Estação Ecológica de Jatí, Luiz Antônio, SP: subsídios para monitoramento ambiental [tese]. São Paulo: Universidade de São Carlos; 2004. 82 p.[Link] [ Links ]
43 Lima JB. Impacto das atividades antrópicas sobre a comunidade de macroinvertebrados bentônicos do rio Cuiabá no perímetro urbano das cidades de Cuiabá e Várzea Grande - MT [tese]. São Paulo: Universidade de São Carlos; 2002. 146 p. [ Links ]
44 Egler M. Utilizando a comunidade de macroinvertebrados bentônicos na avaliação da degradação ambiental de ecossistemas de rios em áreas agrícolas [dissertação]. Rio de Janeiro: Fiocruz; 2002. 147 p.[Link] [ Links ]
45 Fagundes RG, Shimizu GY. Avaliação da qualidade da água do rio Sorocaba - SP através da comunidade bentônica. Rev Bras Ecol. 1997;1(1):63-6.[Link] [ Links ]
46 Cairns J, Pratt JR. A history of biological monitoring using benthic macroinvertebrates. In: Rosenberg DM, Resh VH. Freshwater biomonitoring and benthic macroinvertebrates. New York: Chapman & Hall; 1993. p. 10-27. [ Links ]
47 Reise K. Sediment mediated species interactions in coastal waters. J Sea Res. 2002 Oct;48(2):127-41. Doi: 10.1016/S1385-1101(02)00150-8[Link] [ Links ]
* Article presented at the I Seminar of Environmental and Conservation Research (I Seminário de Pesquisa em Meio Ambiente e Conservação - I SPMAC) organized by the Sciences and Environment Graduate Program (Programa de Pós- Graduação em Ciência e Meio Ambiente - PPGCMA) and the Environmental Computer Simulation Laboratory (Laboratório de Simulação Computacional em Meio Ambiente - LSCMAM) of the Universidade Federal do Pará (UFPA), which took place from 4 to 8 May 2015 in the city of Belém, state of Pará, Brazil. The editors wish to thank the PPGCMA/UFPA and LSCMAM/UFPA partnership and the selection of the Pan-Amazonian Journal of Health for the publication of the excellent studies presented at this event.
7Article originally published in Portuguese (http://dx.doi.org/10.5123/S2176-62232016000300002)
Received: July 06, 2015; Accepted: June 16, 2016











 texto em
texto em 


