INTRODUCTION
Plasmodium falciparum, Plasmodium malariae, and Plasmodium vivax are the main species that cause malaria in Brazil, and the latter parasite is responsible for 80% of the malaria cases registered. Furthermore, 99.7% of reported cases are detected in Brazilian Amazon1,2,3. P. vivax malaria rarely progresses to severe disease; however, studies reinforce the idea that this species may be involved in clinical complications and fatalities, making it a matter of public health concern4,5.
P. vivax sporozoites are covered by circumsporozoite protein (CSP), which is involved in hepatocyte invasion mechanisms and is highly immunogenic6. Genetic variants exist in the central repeat domain of the CSP gene that are characterized as three genotypes, termed VK210, VK247 and P. vivax-like7,8,9. Serological and molecular studies have shown that these genotypes circulate nationwide, mainly in Brazilian Amazon10,11,12,13,14. The VK210 genotype is the most common in the investigated areas, and the VK247 and P. vivax-like genotypes had been found only in mixed infections with other genotypes13,14. However, a decade after the first genotypic study in five different states of Brazilian Amazon, this distribution profile has changed, with the VK247 and P. vivax-like genotypes also detected as single infections in a single area of Pará State15.
Human malaria is transmitted by mosquitoes of the genus Anopheles, which includes 465 recognized species and more than 50 unidentified species16. Transmission occurs in a human-vector-human manner, and the main malaria-transmitting species in Brazil is Anopheles darlingi, although other anopheline species have been detected to be naturally infected with P. vivax and/or P. falciparum and can therefore act as secondary vectors17,18,19,20,21,22.
The sexual reproduction of the parasite occurs during the cycle of the vector, and genetic changes in the plasmodium may occur during this stage23. Additionally, the variability of the repeat region of the CSP gene has also been used to determine infectivity of Plasmodium species in Anopheles24,25. Previous studies using monoclonal antibodies against VK210 and VK247 CSP in non-endemic areas of the Atlantic Forest, São Paulo State, detected the presence of Anopheles infected with both genotypes26. In endemic areas of Brazilian Amazon, 15% of Anopheles were found to be infected with this genotype in Acre State using anti-P. vivax-like antibodies27. In Pará State, it has been observed that the Anopheles aquasalis and An. darlingi species are susceptible to infection by VK210 and VK24728. This demonstrates the importance of studying the genetic diversity of P. vivax in endemic areas. Thus, it is essential to investigate the epidemiology of P. vivax CSP genotypes and to detect naturally infected mosquitoes to understand immunity mechanisms as well as differences in parasite load, drug resistance, transmission dynamics, and natural selective pressure of the parasite among different local communities29,30. The objective of the current study was to investigate the frequency of CSP genotypes in human blood and its correlation with parasitemia, as well as to evaluate the presence of these genotypes in Anopheles in an endemic area of southeastern Pará State, Brazil.
MATERIALS AND METHODS
STUDY AREA
This descriptive epidemiological study was conducted in Municipality of Goianésia do Pará (03°50'33"S, 49°05'49"W) located in Pará State southeastern mesoregion and Paragominas microregion, bordering the Municipalities of Breu Branco, Novo Repartimento, Dom Eliseu, Ipixuna do Pará, Jacundá, and Rondon do Pará. This region has a land area of 7,021 km² and an estimated population of 29,161 inhabitants, of whom 52% are over 14 years old. This area has population density of 4.5 inhabitants/km², and its distance from Belém, the State capital, is approximately 350 km31.
STUDY POPULATION
Patients
The sample was formed by patients living in the localities of Santa Paula, Rouxinol, and Ararandeua that concentrate the highest number of malaria cases in Goianésia do Pará Municipality32 and which sought the healthcare services in their own localities during 2012-2013 and had a positive diagnosis by P. vivax. Thus, a total of 118 blood samples were collected from patients with P. vivax diagnosed by Thick Smear technique and confirmed by nested PCR as described by Kimura et al33. The following individuals were excluded from the study: pregnant women, children under 15 years old, and patients who were infected with other Plasmodium species, displayed mixed infection, or who did not agree to participate. This study was approved by the Ethics Committee for Research on Human Beings of Instituto Evandro Chagas under number 00141/10 - CAAE: 0014.0.0.072.000-10, and participants signed an informed consent form.
Mosquitoes
The adult mosquitoes were collected from the same localities as the patients, and three collections/year (one of 12 h and two of 4 h by locality) were performed. The mosquitoes were identified by dichotomous keys18,34, and their natural infectivity was determined using ELISA protocol35. This method detects natural infection in mosquitoes caused by P. falciparum, P. malariae, and the VK210 and VK247 genotypes of P. vivax.
P. vivax GENOTYPING
DNA was extracted from peripheral blood samples using the Easy-DNATM (Invitrogen, Carlsbad, California, USA) and the QIAamp® DNA Blood Kit (Qiagen, Inc., Chatsworth, California, USA) extraction/purification kits. The P. vivax CSP genotypes were determined using polymerase chain reaction/restriction fragment length polymorphism (PCR/RFLP)36.
RESULTS
P. vivax CSP GENOTYPES
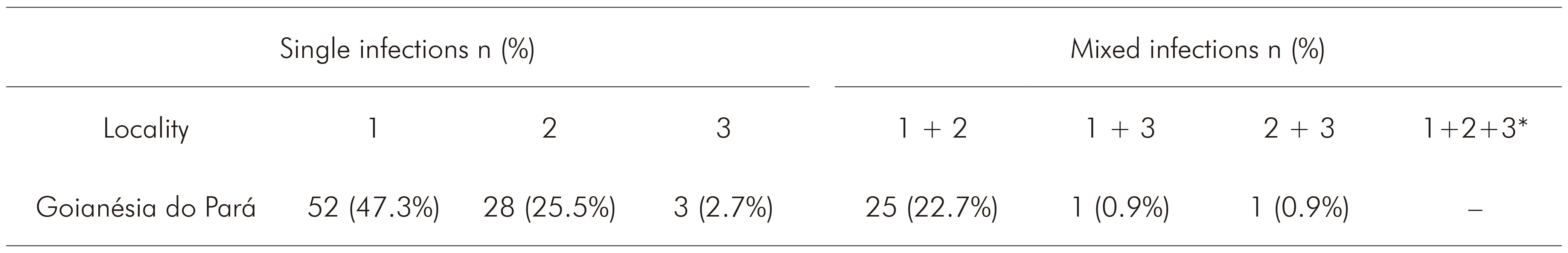
Of the 118 blood samples collected and diagnosed as infected with P. vivax, 110 were successfully amplified and genotyped. The three genotypes were detected both as single and mixed infections (Table 1). There were no mixed infections containing all three genotypes. The most frequent genotype in Goianésia do Pará Municipality was VK210, followed by VK247. Genotyping revealed that 75.5% of the samples were single infections, with only 24.5% mixed infections.
RELATIONSHIP BETWEEN PARASITEMIA AND CSP GENOTYPES
Parasitemia ranged from 5-70,000 parasites/mm³ (geometric mean ± standard deviation: 1,299.95 ± 8:51 parasites/mm³). The mean parasitemia was 569.42 parasites/mm3 (± 10:52) for the VK210 genotype, 2,563.76 parasites/mm3 (± 4.13) for the VK247 genotype, and 3,307 parasites/mm3 (± 5.64) for mixed VK210 and VK247 infection.
The P. vivax-like genotype was excluded from the analysis due to its low frequency. A significant association was observed between the presence or absence of VK247 in the infections and parasitemia. Regardless of whether single or mixed infection was observed, individuals infected with this genotype were associated with the highest parasitemia values (Mann-Whitney, p < 0.001) compared with those without the VK247 genotype (Figure 1).
NATURAL INFECTION OF MOSQUITOES
Of the 369 adult anopheline species found in that Municipality, namely An. darlingi, An. albitarsis s.l., Anopheles triannulatus, Anopheles strodei, Anopheles galvaoi, and Anopheles nuneztovari, only 11 specimens were infected: seven An. darlingi (four with P. falciparum, two with VK210, and one with VK247); three An. albitarsis s.l. (with VK247); and one An. nuneztovari (with VK210).
DISCUSSION
The municipal government of Goianésia do Pará has implemented diagnostic training measures and treatment adherence campaigns, and has introduced insecticide-treated nets to decrease the time between onset of symptoms and the start of treatment, and to reduce or prevent human-vector contact. Due to the significant drop in the number of cases reported in recent years (2011 - 2,856 registered cases, 2012 - 1,136, and 2013 - 192), it is believed that these measures are effective. However, it should be emphasized that clarification of the geographic distribution of genotypes and of mosquitoes naturally infected with different Plasmodium species can provide new opportunities for understanding vector/parasite interaction and the local epidemiology of malaria.
The detection of three P. vivax CSP genotypes circulating in the municipality confirms previous evaluations conducted in Brazilian Amazon in the States of Pará, Rondônia, Amapá, Acre, and Mato Grosso13,15. However, there was no evidence of mixed infections with the three CSP genotypes in Goianésia do Pará Municipality, which contrasts with previous data from Novo Repartimento in Pará State, a Municipality located in the same mesoregion of southeastern Pará15. The VK210 genotype remains the most prevalent, most likely because of the great susceptibility of the An. darlingi vector, which is the most abundant in the region, to this variant28. The P. vivax-like genotype had a frequency of only 2.7% of the genotyped samples; this low frequency could be due to its recent introduction into the region or due to differences in the development of this genotype in the vectors present in the area12,13. Interestingly, the VK247 genotype appeared for the first time as a single infection ten years ago in Municipality of Novo Repartimento15. In this study, this genotype was also found to have naturally infected humans in isolation and was observed in all mosquitoes of the species An. albitarsis and in one An. darlingi specimen.
Another relevant observation is the association of patients infected with the VK247 genotype and the high parasitemia. At the end of the 1990s, Machado and Póvoa13 observed that the VK210 genotype was associated with high levels of parasitemia in the City of Belém (350 km from Goianésia do Pará). This profile change may be related to the evolution of P. vivax. González-Cerón et al37 observed in samples of P. vivax from Mexico, Nicaragua, and Peru that the genetic diversity of the CSP gene is restricted mainly to the central repeat domain and 3'-terminal portion. These authors also stressed that this variation occurs due to changes in the type of nucleotides and number of repeats of the repeat region. The authors noted that the VK247 genotype displays high identity at the carboxy-terminal end with the reported sequence for Plasmodium cynomolgi CSP, and it is possible that the repeat region of VK247 is more stable than VK210. Despite of the association between high parasitemia and the VK247 genotype in this study can not be causal, the data obtained here contribute to the understanding of the molecular epidemiology of P. vivax in Brazil and suggest that the introduction of the VK247 and VK210 genotypes may have occurred at different times according to the endemic area of Brazilian Amazon.
Naranjo-Díaz et al38 showed evidence that the importance and distribution of human malaria vectors may vary depending on location. In fact, these authors observed An. nuneztovari infected with the VK210 and VK247 genotypes in Colombia, showing its importance for malaria transmission in areas with anthropic intervention38. This pattern was also observed in Brazil, where An. darlingi was found to be infected only with VK247 in District of Lourenço, Amapá State39. Moreover, in Municipality of Marabá, another mesoregion of southeastern Pará State, An. darlingi was found to be infected by P. falciparum and VK247, whereas An. albitarsis was infected with both VK210 and VK247 genotypes40. In turn, there was a 1.22% infection rate in Acre State for VK247 in the Anopheles oswaldoi mosquito24, the main malaria vector in the region41. In this descriptive study, the VK247 CSP genotype was detected in one mosquito An. darlingi and in three An. albitarsis, corresponding to 36.4% (4/11) of infection. As these two species play an important role in malaria transmission in Brazil and because this variant has been detected separately in different locations in this mesoregion of Pará, it was hypothesized in this study an increase in the number of cases of this genotype in the region. This new evidence should be investigated in other locations where different species of anopheline mosquitoes are the main vector, such as An. aquasalis in Amazon Region20 and Anopheles bellator and Anopheles cruzii in outside Amazon region3, to determine whether other species may facilitate the transmission process by carrying different CSP genotypes.
In general the distribution profile of CSP genotypes in other countries of Latin America is different from that observed in Brazil. Thus, the possibility of an outbreak of vivax malaria cases by VK247 in Brazil cannot be ruled out. This could lead to higher parasitemia as well as more severe clinical conditions. Although the VK247 variant is ancestral and its CSP repeat region is more stable than that of VK21042, the distribution of this genotype can be quite heterogeneous in other regions of Brazilian Amazon, most likely due to the absence or presence of VK247 polymorphisms in different locations and this fact may be related to changes in the geographical distribution profile of this variant37,43.
Another hypothesis that reinforces this idea is related to the immune response. The VK210 genotype is more immunogenic than VK247, which leads to high immune responses against this genotype and facilitates the selection process of VK247 sporozoites. The possible presence of anti-VK210 antibodies may therefore limit the production of VK210 sporozoites and result in a lower frequency of this genotype in some species of mosquito44. This may also impact the parasite load in infected individuals. These variations can occur due to physiological incompatibility between host/parasite, defense mechanisms of the mosquito such as the destruction of ookinetes blocking the development of oocysts in the mosquito, or ecological and evolutionary factors that can contribute to the divergence or restriction of gene flow among parasite strains adapted to different local vectors45,46,47. It is noteworthy that the detection of CSP in vectors only occurs when the parasite reaches the sporoblast stage48,49, and for this reason it is possible that the absence of CSP expression in a parasite or even the vector's immune response against this protein may have influenced the detection of genotypes in mosquitoes.
CONCLUSION
Although the VK210 genotype remains the most prevalent in Brazil, a new evidence reveals a strong adaptation of the VK247 variant in southeastern Pará, as well as the association of this genotype with high parasitemia. The species An. darlingi, An. albitarsis and An. nuneztovari play an important role in the transmission of these genotypes in the study area. However this is the second time that An. albitarsis has been found in a natural infection with the VK247 genotype in Pará State, and it may be the main vector in the spread/selection of this genotype. This may therefore present a public health concern because it raises the possibility of a resurgence of vivax malaria epidemics in susceptible communities.












 Curriculum ScienTI
Curriculum ScienTI