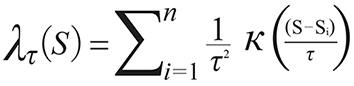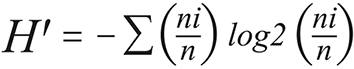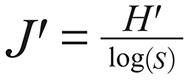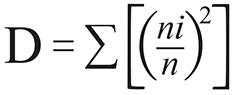INTRODUCTION
The American tegumentary leishmaniasis is a vector-borne disease caused by Leishmania protozoals and transmitted by phlebotomine sand flies (Psychodidae: Phlebotominae). The disease represents an important problem of public health in rural and urban areas in Brazil. High incidence rates of tegumentary leishmaniasis had been computed over the last decade (2001-2010) and more than 30,000 new occurrences were reported in 20101, mostly (~95%) represented by cutaneous leishmaniasis (CL) and the smaller proportion by mucosal or mucocutaneus leishmaniasis (ML).
Different species of phlebotomine sand fly are able to transmit CL to humans. The community composition and richness of sand flies result of ecological interactions and local geographic aspects2,3, then environmental changes affect the abundance, diversity, behaviour and habits of the different species within a community4.
Some environments become highly favourable to the CL vectors development and disease transmission depending on the local influences5,6. At least three factors determine the vectorial capacity: density of the species, ability to colonize humans changed environments and the competence for feeding human blood7.
Additionally, the Leishmania species distribution and seasonality are reflections of the events affecting their vectors8. For each Leishmania pathogen there is one or more species of sand fly vectors playing a role on the protozoal transmission to humans9,10. However, the importance of each CL vector varies with the different endemic areas and climatic seasons.
The epidemiological surveillance and phlebotomines ecology understanding are essential to guide preventive measures especially in areas of high biological diversity where continuous environmental changes take place9,11.
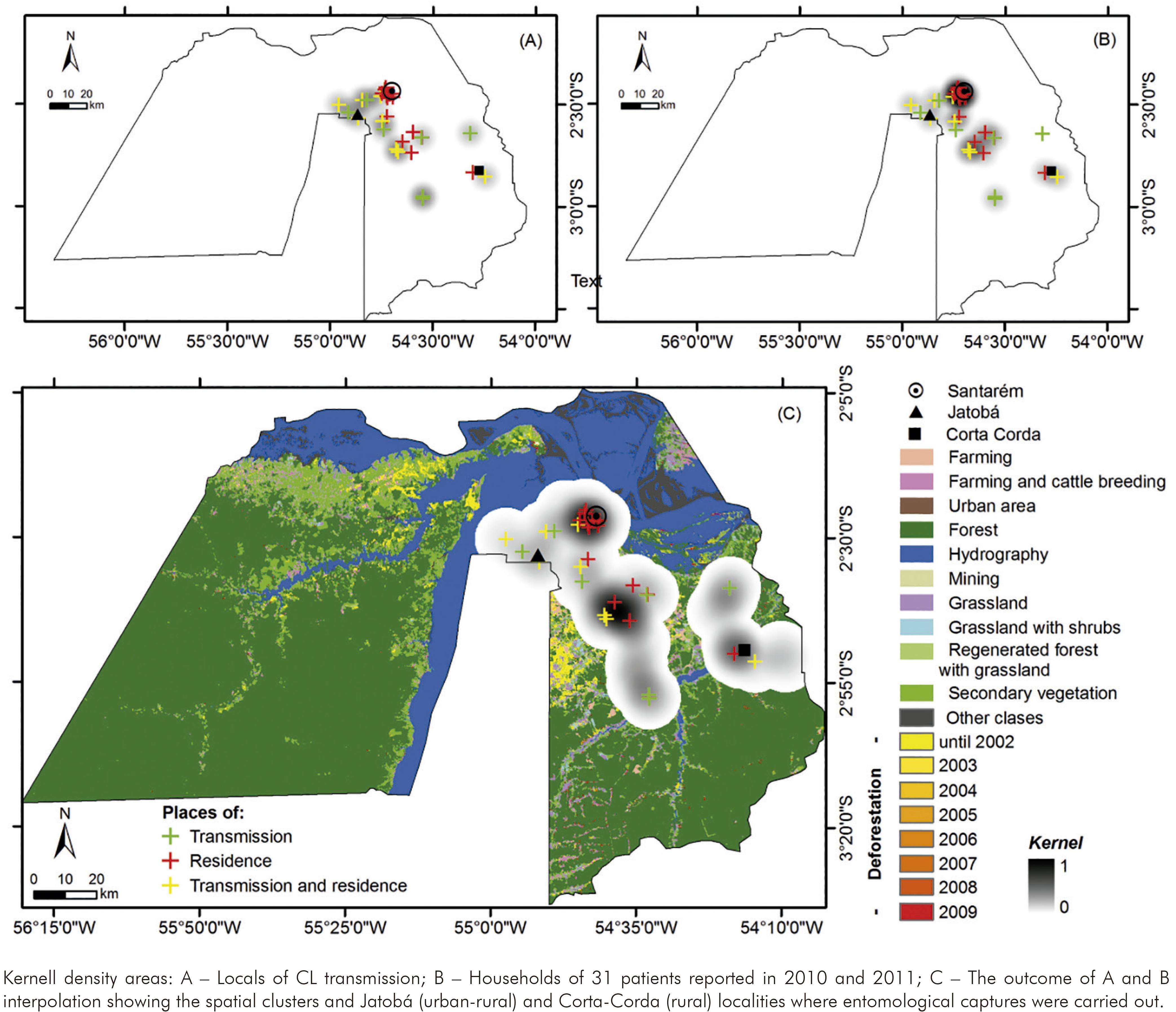
The Municipality of Santarém (lower Amazon Region) is the main centre reporting new CL occurrences on western Pará State. Autochthonous CL reports were firstly investigated throughout 2010 and 2011, and clusters were identified with spatial analysis tools5,12,13. Then it was described the fauna and studied the ecology of phlebotomine sand fly communities in these risk zones by calculating ecological indexes.
MATERIALS AND METHODS
For this entomologic study, recommendations were followed of the Instituto Evandro Chagas (IEC) Human Research Ethics Committee (0019/2012). Additionally, it was used a database of CL records previously obtained with ethics approval of this same committee (CAAE 0030.0.072.000-09).
The Municipality of Santarém (02º26' S, 54º42' W) is a development centre on western Pará State. The temperature (25-28° C) and relative air humidity (86%) is frequently high. The rainfall is over the whole year, despite it is more frequent from December to June (rainy season) rather than in the other months (dry season). A dense ombrophilous forest forms the natural vegetation cover, but large areas with intense agricultural activities (mainly soy culture) provide a secondary vegetation cover. Several businesses have a direct impact on the local environment, such as the Curuá-Una hydroelectric dam14.
The population was 294,580 inhabitants in 2010 and the territory extension was 24,154 km² until 2013, when it was reduced to 17,898 km². The incidence rate of CL was 42 and 66 new cases for 100,000 inhabitants in 2010 and 2011 respectively15.
The database was composed of 102 entries including autochthonous (47) and allochthonous (55) occurrences of CL reported in Santarém, during 2010 and 2011.
Thirty-one records of autochthonous CL reports were selected, including patients infected with Leishmania sp. (8, 26%), Leishmania (Viannia) sp. (9, 29%), Leishmania (Viannia) braziliensis (7, 23%), Leishmania (Viannia) shawi (4, 13%), Leishmania (Viannia) lainsoni (1, 3%), Leishmania (Viannia) naiffi (1, 3%) and a putative hybrid of L. (V.) braziliensis/guyanensis (1, 3%). Etiologic agents were previously identified by means of PCR and/or sequencing16. The database had several information about the patient, including the putative CL transmission locals and households (place of the event). The excluded records had no information about places of the event (14/47) or these places were inaccessible by road (2/47).
In order to get the GPS coordinates (Global Position System, Garmin(r) Colorado 400 T and 76 CSx) in the places of the event, it was used the ArcGIS 10 (Environmental System Research Institute Inc., Redlands, California, USA) and applied the probability density function estimated by the Kernel method as follow17:
Where 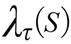 is the estimated value in a region;
is the estimated value in a region; 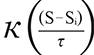 means the Kernel function; S...Si
are the places of the event in a radius of influence (τ) and centre (S).
means the Kernel function; S...Si
are the places of the event in a radius of influence (τ) and centre (S).
A satellite image at 250 m spatial resolution and 10 km radius highlighted the risk zones (clusters), deforestation (2002-2009) and land uses (Amazon Deforestation Monitoring Project, PRODES - TerraClass Project, INPE, 2011).
The entomological capture units considered were environments with potential sand fly blood sources (animals and humans): indoors, chicken sheds and surrounding forest. Then four capture units in each spatial cluster (SC) or risk zone were defined. Three were represented by houses and their respective chicken sheds in the peridomiciliary areas (SC1: -2,569 -54,861 Datum SAD 69, altitude of 83-107 m; SC2: -2,831 -54,294, altitude of 35-72 m). A fourth unit was a locality in the surrounding forest (SC1: -2,551 -54,860 Datum SAD 69; 141 m altitude; SC2: -2,849 -54,299 Datum SAD 69; 64 m altitude).
Six CDC light traps were placed at a height of 1.5 m in the peri- and intradomiciliary areas of three houses from 6 p.m.-6 a.m. for three nights and a Shannon trap installed in the surrounding forest from 7 p.m.-9 p.m. for a single night. This sampling regime was carried out on two occasions, once each in the rainy (March) and dry (September) seasons of 2012. The choice of the capture units followed criteria earlier established9. The captured phlebotomines were segregated by trap and environment and then identified by morphology18.
It was used the PAST v2.03 (PAlentological STatistic) for calculating the ecological indexes and comparing attributes among samples. The standard deviation and confidence interval were computed by permutation and bootstrap (α = 0.05). The following indexes were calculated:
In the equations above n is the overall number of individuals, ni is the number of individuals of a given species (i) in each environment and S is the number of species.
It was also performed the chi-square test (χ2) for comparing the frequencies of male and female phlebotomines in the rainy and dry seasons (α = 0.05).
RESULTS
The figure 1 shows two Kernel density areas or risk zones in Santarém based on the CL occurrences density in 2010 and 2011. The bigger cluster is overlapping an urban-rural area including a locality called Jatobá, where most patients were infected. The smaller one covers a rural area on southeastern Santarém, where is found Corta-Corda locality. Entomological surveys were carried out during the rainy and dry seasons in both localities.
A total of 417 phlebotomine sand flies was captured in urban-rural (58%, 243) and rural (42%, 174) areas, most females (75%, 312). The sand flies frequency did not vary in the rainy (54%, 226) and dry seasons (46%, 191). A higher female proportion was found when used the Shannon trap irrespective of the season (p < 0.0001) and when CDC light traps were used in the dry season only (p = 0.0003). In the rainy season, no significant difference in sex proportion was found in the phlebotomine sample captured with CDC light traps (p = 0.52). Ten genera in the sample were identified: Bichromomyia, Evandromyia, Lutzomyia, Micropygomyia, Nyssomyia, Psathyromyia, Psychodopygus, Sciopemyia, Trichopygomyia and Trichophoromyia (Table 1). Vectors of the same Leishmania species causing CL were found in both urban-rural and rural areas: Psychodopygus complexus, Nyssomyia anduzei, Bichromomyia flaviscutellata, Psychodopygus davisi and Nyssomyia antunesi. It was also found Lutzomyia longipalpis, a visceral leishmaniasis (VL) vector, in urban-rural area (Table 2). For calculating ecological indexes, It was used the species frequency in different environment and seasons (Table 1).
Table 1 - Phlebotomine species abundance and frequency in different environments associated to the ecological indexes in two surveys during the rainy (March) and dry (September) seasons of 2012 in urban-rural (Jatobá) and rural (Corta-Corda) areas of Santarém, Pará State, Brazil
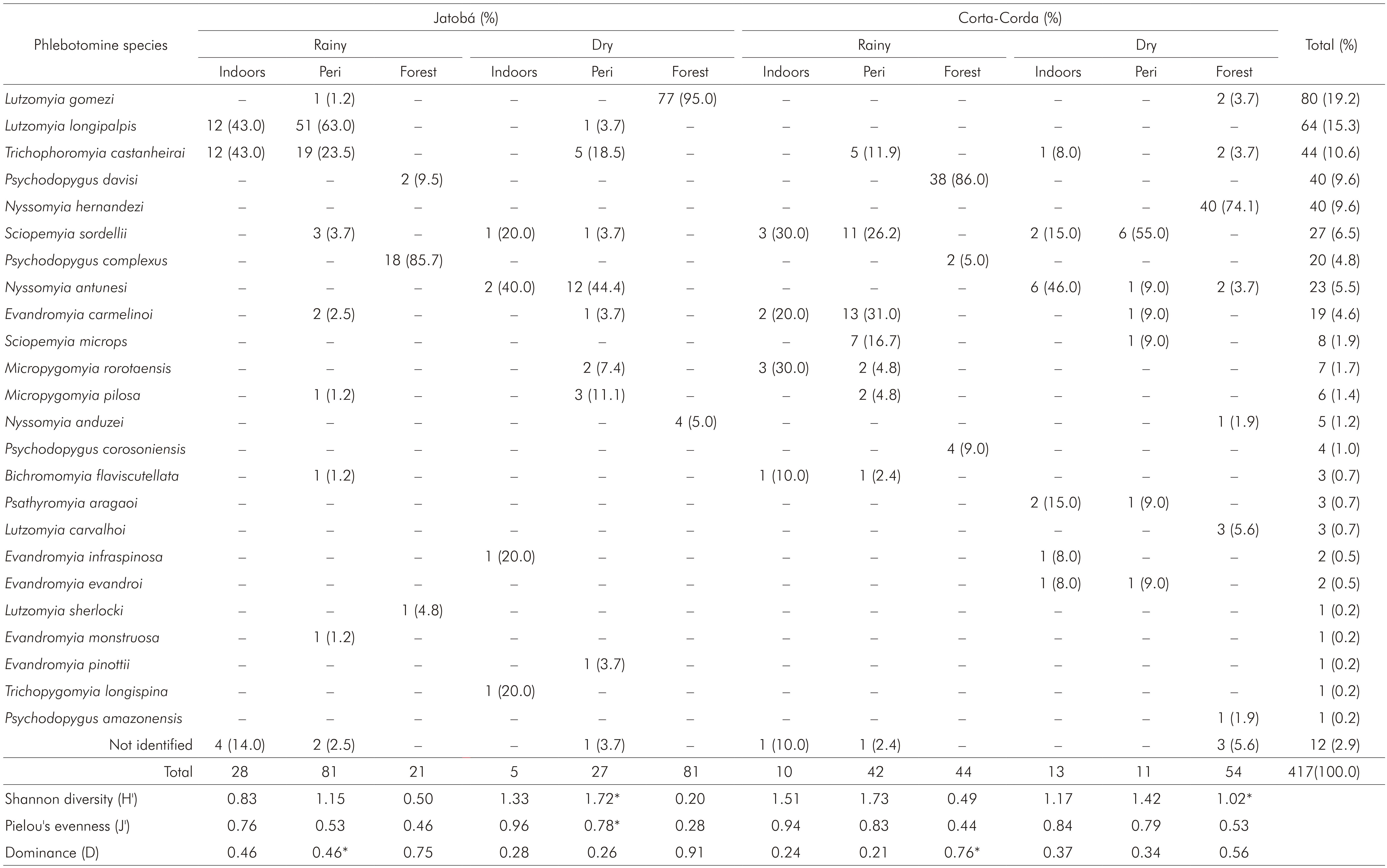
Peridomiciliary areas (chicken sheds); * Significantly higher between seasons (p < 0.05); Conventional signal used: - numerical data equals zero.
Table 2 - The six phlebotomine sand flies species in the sample incriminated and/or confirmed as vectors of epidemiologically important Leishmania species in Amazon Region

The Shannon diversity ranged 0.2-1.73, increasing significantly during the dry season in the forest of the rural area and in the chicken sheds of the urban-rural area. For the other environments, no significant difference between seasons was described.
Pielou's evenness ranged 0.28-0.96, with no season variation in rural area. Nevertheless, the J' values increased significantly during the dry season in urban-rural peridomiciliary areas.
The dominance ranged 0.21-0.91 and had the highest values associated to the forest (0.56-0.91). Potential vectors of CL were dominant species in the forest of the urban-rural area, where Ps. complexus was dominant, and in the forest of the rural farm area, where the dominant species was Ps. davisi, both in the rainy season. These CL vectors alternated the dominance behaviour in different seasons with other phlebotomine species without epidemiological importance, Lutzomyia gomezi and Nyssomyia hemandezi, respectively dominant in urban-rural and rural areas during the dry season, when the CL vectors were less frequent or entirely absent. A significant season variation concerning to the D index was only observed in the rural area. No Lu. longipalpis was found in the rural area, but was present in the urban-rural peridomiciliary areas as a dominant species during the rainy season. Lu. longipalpis shared the dominance with Lutzomyia castanheirai indoors. The D value associated to Lu. longipalpis decreased significantly though the dry season, when this species was not found indoors (Table 1).
DISCUSSION
Through the last decades, Santarém undergoes continuous environmental changes due to a fast economic development specially based on its natural richness and agricultural activities27. In despite of the high incidence of human CL with several Leishmania species causing the disease, the ecology of CL vectors in this Amazonian municipality is poorly known. In this study, twenty-four species of phlebotomines were identified including several potential vectors of Leishmania pathogens to humans, all compatible with the diversity of parasites infecting 31 patients who lived and worked in the risk zones. Surveys in areas of other Brazilian biomes had reported lower richness in samples up 10 times larger than those carried out in this study. Studies carried out in areas of Cerrado and Pantanal biomes recognized only eight phlebotomine species within a sample of 3,946 phlebotomines monthly captured with CDC light traps in the course of one year. Two of the species were epidemiologically important - Nyssomyia whitmani and Lu. longipalpis - being the second highly abundant28. A survey in Bahia State, which assembles Atlantic forest, Caatinga and Cerrado, sampled 3,000 phlebotomines of 14 species, including only one CL vector, Ny. whitmani29.
The ecological indexes vary slightly as the sample size increases thus they are feasible to calculate even upon small samples, as used in this study30,31. The Shannon diversity index, higher as the diversity increases and lower as the proportion of rare species increases, despite of the large interval (H' = 0-10), usually ranges 1.5-3.5, in this study it ranged 0.2-1.72. The microenvironments that presented variations in diversity between seasons were peridomiciliary locals of the urban-rural area (dry: 1.15; rainy: 1.72) and surrounding forest of the rural area (dry: 0.49; rainy: 1.2). This rural area has agricultural activities and a hydroelectric dam of Curuá-Una river. The phlebotomine diversity and evenness there ranged respectively: H' = 0.49-1.72 and J' = 0.44-0.96. Those values represent more diversity and similar evenness if compared to the environments around another hydroelectric dam in southern Brazil (H' = 0.3-1.31; J' = 0.2-1.0), Paranapanema river, Paraná State32, where Atlantic forest is the predominant bioma.
The Pielou's evenness index (J') or equivalence of species abundance is strongly influenced by the climate variations and environmental changes. When the J' value is high, there is no dominant species and the dominance index is low. In this study, the Pielou's index increased significantly between seasons (rainy: 0.53; dry: 0.78) only in peridomiciliary areas of Jatobá, where the dominance was associated to Lu. longipalpis, the vector of L. (L.) infantum, which causes VL. The correspondent D values (rainy: 0.46; dry: 0.26) indicated that half of the phlebotomine community in this microenvironment is composed, during the rainy, by Lu. longipalpis, beside more diversity in the peridomiciliary areas (H' = 1.15) than indoors (H' = 0.83). Indoors, Lu. longipalpis shared dominance with Th. castanheirai, species with no epidemiological importance until now.
The diversity is a positive attribute of natural communities. Those with more diversity are more stable and less susceptible to environmental disturbances33, but deep environmental changes arise imbalances and can lead important species to be highly abundant in a given area. The dominant species are able to turn into competent Leishmania vectors to humans. Consequently, outbreaks of CL can take place5,6. In Santarém, Jatobá is a risk zone for both CL and VL.
Dominant species were found in the forest of the urban-rural area as well. High D values were described in both seasons (rainy: 0.75; dry: 0.91), respectively associated to Ps. complexus and Ps. gomezi, being an important vector of L. (V.) braziliensis. The species Ps. complexus usually predominates until 200 m above the sea level34. The elevated abundance of this species during the rainy season seems to be common in the western Pará State35. In the forest of the rural area, D value increased significantly through the rainy season only (0.76), what was associated to Ps. davisi, a potential vector of L. (V.) braziliensis and L. (V.) naiffi (Table 2).
The CL vectors sampled were well-matched with the findings of Leishmania pathogens that infected patients. The most frequent etiologic agent among the patients was L. (V.) braziliensis, for which it was found more than one potential vector in the sample. Another important species playing the role of L. (V.) braziliensis vector in Pará State was Ps. wellcomei36, which is morphologically indistinguishable from Ps. complexus. Nevertheless, this species lived in the range of 200-700 m above the sea level34,36, so it was supposed that this small sample had only specimens of Ps. complexus, since phlebotomines were captured in altitudes less or equal 141 m. Anyway, Ps. wellcomei could be also represented in larger samples in low altitudes in despite of a lower frequency if compared to Ps. complexus.
However, Ps. complexus was entirely absent in the rural area, where Ps. davisi was the dominant species in the forest through the rainy season. Other studies have been reported the natural infection of Ps. davisi with L. (V.) naiffi21 and L. (V.) braziliensis20 in different localities of Amazon Region. On the other hand, it was also found L. (V.) naiffi which infected the patients in the study area. Although people living in rural areas are exposed to both vectors when get into the forest, Ps. davisi shall have epidemiological importance in the rural area (around the Curuá-Una river and hydroelectric dam) during the rainy season, while Ps. complexus would be the main CL vector in Jatobá (urban-rural and ecotourism area).
Despite the importance of one or more species in a phlebotomine community can be more easily detected when observed the dominance index, it is necessary to register all potential vectors of Leishmania pathogens to humans in the sample. In addition to the dominant species mentioned above, Bi. flaviscutellata, vector of L. (L.) amazonensis was also sampled.
In contrast to Ps. complexus, Bi. flaviscutellata do not bite humans frequently, preferring animals. Traps with rodents such Disney traps strongly attracted Bi. flaviscutellata25,37,38,39, but in captures using CDC and Shannon light traps this species is poorly represented. However, Bi. flaviscutellata was one of the five most abundant species captured in other survey carried out in rural areas of Santarém40. In this study, none of the patients living and working in the risk zones (SCs) had diagnosed a L. (L.) amazonensis infection. But there was no information about the subgenus neither the Leishmania species causing CL in 26% patients, who could be possibly infected by L. (L.) amazonensis.
Interestingly, although Bi. flaviscutellata is a known vector of L. (L.) amazonensis, the natural infection of this phlebotomine species with L. (V.) guyanensis, that can induce mucosal lesion similar to L. (V.) braziliensis26 had been also reported in French Guiana41. In view of this arguments, it was considered that the presence of Bi. flaviscutellata indoors in the rural area and in the chicken sheds in both urban-rural and rural areas always in the rainy season, in despite of the low frequency, raising questions about its epidemiological importance in the SCs of CL.
The main vector of L. (V.) guyanensis in Amazon Region is Nyssomyia umbratilis, which was not found in the sample. But Ny. anduzei, a secondary vector of this parasite in Amazonas State, Brazil24 had been found. One of the records of CL cases reported a patient infected with a putative hybrid of L. (V.) braziliensis/L. (V.) guyanensis. Recently, it was reported a putative hybrid involving these two Leishmania species in Santarém16,42. Hybrids are an important aspect of the Viannia subgenus whose presence would indicate the possible generation of epidemiologically important genotypes, since they have been described in focus of mucocutaneous leishmaniasis43. The Leishmania ability of infecting different phlebotomine species could modulate genetic changes in the parasite, so the vector role in the epidemiology and pathogenesis of Leishmania infections needs to be more studied44.
Ny. antunesi (Table 2), the putative vector of L. (V.) lindenbergi in Belém23, Pará State was also found in the sample. Ny. antunesi was the most abundant species captured in the dry season, both indoors and in the peridomiciliary areas. Although the high abundance described for this species, there is no evidence of L. (V.) lindenbergi circulating in the same area. The PCRs (Hsp70-234 and ITS1) using samples of patients living in the SCs of CL were unable to discriminate L. (V.) lindenbergi and L. (V.) guyanensis16,45.
In general, the current study showed a positive relation between potential CL vectors and the etiologic agents infecting the patients. However, further studies on molecular entomology searching natural infection by Leishmania species in phlebotomines are necessary especially in such areas with high biological diversity.
In despite of the high incidence of CL in Santarém, both VL and CL are public health problems there. High abundance and dominance of Lu. longipalpis were detected in the peridomiciliary areas of Jatobá during the rainy season only. This vector was absent in the rural area in both seasons. The high abundance of Lu. longipalpis in Santarém outskirts is already known46. This event seems to be consequence of the space occupation in Jatobá, where prevails the ecotourism, the flow of people is intense, houses are nestled deep in the secondary forest and there are no available water and sewerage service favours Lu. longipalpis development47. This species shows opportunistic feeding behavior and ability to colonize several environments, especially those continuously impacted by anthropogenic changes48. The domestic animals and respective shelters provide to Lu. longipalpis several sources of feeding, resting and oviposition46,49,50. These variables act as conditioning factors of the VL vector presence near the houses or even indoors, favouring their contact with humans51.
CONCLUSION
It was observed high diversity of phlebotomine species in both SCs and significant dominance variations depending on the species behaviour and adaptation following the environmental changes. Additionally, the entomological findings of CL vectors supported Leishmania species diversity in patients living in the SCs of CL in the Municipality of Santarém, representing the importance of spatial analysis methods for guiding entomological surveys.
Ps. complexus and Ps. davisi are probably the main CL vectors in urban-rural and rural areas, respectively, but the epidemiological importance of each species in both communities could vary in the different climatic seasons. Other potential CL vectors in the sample (Ny. antunesi, Bi. flaviscutellata and Ny. anduzei) would play a secondary role on dermotropic Leishmania transmission, but their epidemiological importance needs to be further investigated as well. The urban-rural area in Santarém is also a risk zone for VL transmission. It is necessary to investigate natural infection by Leishmania in sand fly species and understand better their seasonality and risk factors for Leishmania pathogen transmission.