Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Pan-Amazônica de Saúde
versión impresa ISSN 2176-6215versión On-line ISSN 2176-6223
Rev Pan-Amaz Saude vol.11 Ananindeua 2020 Epub 20-Ago-2020
http://dx.doi.org/10.5123/s2176-6223202000468
ORIGINAL ARTICLE
Effectiveness of treatment with direct-acting antiviral drugs in patients with hepatitis C treated at a referral center in the Pará Sate, Brazil, from 2017 to 2019
1 Universidade do Estado do Pará, Belém, Pará, Brasil
2 Universidade Federal do Pará, Belém, Pará, Brasil
3 Fundação Santa Casa de Misericórdia do Pará, Grupo do Fígado, Belém, Pará, Brasil
OBJECTIVE:
To evaluate the effectiveness and tolerability of treating hepatitis C with direct-acting antivirals in patients with chronic hepatitis C treated at the Hepatology Outpatient Clinic of Fundação Santa Casa de Misericórdia do Pará, in Belém, Pará State, Brazil.
MATERIALS AND METHODS:
Cross-sectional study with 305 patients treated with sofosbuvir (SOF), daclatasvir (DCV) or simeprevir (SMV) from May 2017 to March 2019. The medical records of 250 patients who completed treatment during this period were analyzed, of which demographic and clinical data were evaluated.
RESULTS:
There was a predominance of males (50.40%), mean age of 61.21 years old and from Belém (69.20). Most of them (54.00%) had cirrhosis, and 40.80% reported previous treatment. Genotype 1 was found in 73.60%, and genotype 3 in 23.20% of cases. The standard treatment regimen was SOF, DCV and ribavirin (RBV) for 12 weeks. The rate of sustained virologic response (SVR) was 97.2%. n Four non-responders from genotype 3 and three from genotype 1, using three schemes with SOF+DCV+RBV for 12 weeks; two regimens with SOF+DCV for 12 weeks; a SOF+DCV regimen for 24 weeks; and a regimen with SOF+SMV for 12 weeks.
CONCLUSION:
The results of this study showed a predominantly urban population, most of them were men and cirrhotic, with a predominance of genotype 1. It is important to notice the good tolerance and high effectiveness of the new direct-acting antivirals with an overall SVR rate of 97.2%.
Keywords: Chronic Hepatitis C; Antivirals; Sofosbuvir; Simeprevir; Ribavirin; Combination of Drugs
INTRODUCTION
Hepatitis C virus (HCV) infection is a serious public health problem worldwide. The World Health Organization (WHO) estimates that 71 million people live with the chronic form of HCV infection (global prevalence of 1%), and that 1.75 million new cases occur annually1.
In Brazil, 359,673 cases were reported between 1999 and 2018. In the analysis of the distribution of cases by regions, 63.1% occurred in the Southeast, 25.2% in the South, 6.1% in the Northeast, 3.2% in the Midwest and 2.5% in the North. The Pará State was in the third place among the states of the Northern Region, with 2,659 confirmed cases in that period2.
The disease has a high chronic rate, around 50-80%, considered as the permanence of the infection for a period similar to or greater than six months. The chronic form can cause serious liver problems, such as the progression to advanced stages of fibrosis and cirrhosis, in approximately 20% of cases, and liver cancer, complications that can increase the possibility of death3. For this reason, treatment should be recommended for every patient diagnosed.
The treatment of hepatitis C went through several stages until reaching the current standards. Previously, the use of interferon-α, ribavirin and pegylated interferon was recommended in several regimens, depending on the virus genotype4. Such therapeutic regimens had a long duration, caused several adverse events of difficult tolerance, such as fatigue, depression and exacerbation symptoms of autoimmune diseases, and had low efficacy, with sustained virological response rates (SVR) around 50%5. As of 2011, clinical trials have shown that the association with direct-acting antiviral agents was an effective strategy for treatment, increasing the rate of SVR. Thus, boceprevir and telaprevir were options added to the treatment of hepatitis C, in association with ribavirin and pegylated interferon, creating a triple scheme that, however, continued to present undesirable effects6. As of 2014, a second generation of direct-acting antivirals has been used, represented by sofosbuvir, daclatasvir and simeprevir, which is a milestone in the treatment of hepatitis C, because these new drugs presented SVR rates around 90% and allowed a therapy without the need to use interferon, thus drastically reducing the adverse events previously found, simplifying and reducing the treatment duration5,7.
In Brazil, since 2015, the Ministry of Health, through the Clinical Protocol and Therapeutic Guidelines (PCDT) for Hepatitis C and Coinfections, recommends the use of new direct-acting antiviral agents. The drugs were sofosbuvir, simeprevir and daclatasvir8. Subsequently, new PCDTs included new drugs to the therapeutic arsenal and removed others. Currently, the drugs proposed by PCDT 20199 are: daclatasvir, sofosbuvir, ledipasvir, elbasvir, glecaprevir and velpatasvir.
The efficacy of the new drugs has been proven by clinical trials conducted in populations and several locations around the world. However, even with successful experiences in many locations, there is a great need for Brazilian studies, and particularly regional studies, after the implementation of the new treatment for hepatitis C by the Unified Health System (SUS), with the aim of researching the impact and benefit also in the Brazilian population and, specifically, Amazonian one. Thus, the current study aimed to evaluate the impact of treatment for hepatitis C, proposed by the PCDT for hepatitis C and coinfections 201710, regarding the rate of SVR of patients with chronic hepatitis C, at the Hepatology Outpatient Clinic of the Fundação Santa Casa de Misericórdia do Pará (FSCMPA), a reference of treatment in the Pará State.
MATERIALS AND METHODS
In this cross-sectional study, data were collected from 305 medical records of patients with chronic hepatitis C, attended at the Hepatology Outpatient Clinic of FSCMPA, in Belém, between May 2017 and March 2019. Data collection followed the rules of Resolution 466/2012 of the National Health Council and was authorized by the Research Ethics Committee of FSCMPA, under opinion no. 2,085,121, on May 26, 2017.
Patients over 18 years of age who completed treatment between May 2017 and March 2019 were included in the sample, using the agents sofosbuvir, daclatasvir or simeprevir, associated or not with ribavirin with any viral genotype, stage of fibrosis or previous experience with alternative regimens. Patients with no clinical follow-up data were excluded from this study. The proposed treatment regimen was the responsibility of the physician from FSCMPA, according to the drugs available by SUS and PCDT 2017, with no participation of researchers in this decision. Demographic and clinical data were evaluated, such as gender, age, municipality, degree of fibrosis, previous treatment experience, genotype and viral load, treatment regimen, presence of adverse events and the scheme effectiveness according to SVR, through its own research protocol. In agreement with the 2017 PCDT, SVR was considered as the absence of HCV-RNA on polymerase chain reaction (PCR) examination at the 12th or 24th week after the end of drug treatment. real time - reverse transcription polymerase chain reaction (RT-PCR) (HCV-RNA) (Abbott®) and HCV genotyping by sequencing or INNO-LiPA® techniques were carried out at the Laboratório Central of Pará. The degree of fibrosis was measured by liver biopsy, recorded in medical records, or noninvasive methods such as the AST to Platelet Ratio Index (APRI), the Fibrosis-4 score (FIB-4) and the FibroScan® elastography test, correlated with the METAVIR score.
The information obtained from this study was organized and submitted to statistical analysis by protocol of the chi-square test with Yates' correction. Microsoft Office Excel 2010 and BioEstat v5.3 softwares were used for data analysis.
RESULTS
Among the 305 medical records, 55 were excluded due to the absence of information related to clinical follow-up, resulting in a final sample of 250 patients.
Clinical and demographic information of the population studied, according to the proposed treatment regimen, is shown in Table 1. Among the 250 patients analyzed, 126 (50.40%) were male. The mean age was 61.21 years; 155 (62%) were 60 years of age or older, 92 (36.80%) between 40 and 59 years old and three (1.20%) were under 40 years old. The majority, 173 (69.20%), lived in Belém. Regarding clinical data, 93 (37.20%) had viral load prior to treatment greater than 800,000 IU/mL; 175 (70,00%) received treatment with sofosbuvir and daclatasvir, 69 (27.60%) sofosbuvir and simeprevir and six (2.40%) with other schemes. Genotype 1 was the most frequent (73.60%), followed by genotype 3 (23.20%) and genotype 2 (3.20%). Of the total number of patients analyzed, 102 (40.80%) had some previous treatment experience and 135 (54.00%) were carriers of liver cirrhosis. Ribavirin was added in 121 (48.40%) cases; and 212 (84.80%) patients had a duration of treatment of 12 weeks. The overall SVR rate was 97.20%, with therapeutic failure in only seven (2.80%) Cases. Adverse events were reported by 54 (21.60%) individuals.
Table 1 - Clinical and demographic characteristics by treatment regimen, of patients with chronic hepatitis C treated with direct-acting antiviral agents at FSCMPA in Belém, Pará State, Brazil, between May 2017 and March 2019
| Variables | SOF+DAC±RBV | SOF+SMV±RBV | Other | Total | p-value* | ||||
|---|---|---|---|---|---|---|---|---|---|
| Average | Min.-max. | Average | Min.-max. | Average | Min.-max. | Average | Min.-max. | ||
| Age | 61,55 | 38-81 | 60,21 | 30-76 | 62,66 | 48-73 | 61,21 | 30-81 | 0,559000 |
| N = 175 | % | N = 69 | % | N = 6 | % | N = 250 | % | ||
| Sex | |||||||||
| Male | 91 | 52,00 | 35 | 50,72 | 3 | 50,00 | 126 | 50,40 | 0,857519 |
| Female | 84 | 48,00 | 34 | 49,28 | 3 | 50,00 | 124 | 49,60 | |
| Genotype | |||||||||
| 1 total | 116 | 66,29 | 68 | 98,55 | - | - | 184 | 73,60 | < 0,00001 |
| 1 not specified | 30 | 25,86 | 12 | 17,65 | - | - | 42 | 22,83 | 0,963072 |
| 1a | 27 | 23,28 | 17 | 25,00 | - | - | 44 | 23,91 | 0,091977 |
| 1b | 59 | 50,86 | 39 | 57,35 | - | - | 98 | 53,26 | 0,001065 |
| 2 | 3 | 1,71 | - | - | 5 | 83,33 | 8 | 3,20 | NA |
| 3 | 56 | 32,00 | 1 | 1,45 | 1 | 16,67 | 58 | 23,20 | 0,000001 |
| Viral load (UI/mL) | |||||||||
| < 800.000 | 105 | 60,00 | 49 | 71,01 | 3 | 50,00 | 157 | 62,80 | 0,108294 |
| ≥ 800.000 | 70 | 40,00 | 20 | 28,99 | 3 | 50,00 | 93 | 37,20 | |
| Previous treatment | |||||||||
| Yes | 74 | 42,29 | 27 | 39,13 | 1 | 16,67 | 102 | 40,80 | 0,652236 |
| No | 101 | 57,71 | 42 | 60,87 | 5 | 83,33 | 148 | 59,20 | |
| Fibrosis | |||||||||
| F0-F3 | 73 | 41,71 | 39 | 56,52 | 3 | 50,00 | 115 | 46,00 | 0,036585 |
| F4 | 102 | 58,29 | 30 | 43,48 | 3 | 50,00 | 135 | 54,00 | |
| Duration of treatment | |||||||||
| 12 weeks | 137 | 78,29 | 69 | 100,00 | 6 | 100,00 | 212 | 84,80 | NA |
| 24 weeks | 38 | 21,71 | - | - | - | - | 38 | 15,20 | |
| Addition of RBV | |||||||||
| Yes | 98 | 56,00 | 17 | 24,64 | 6 | 100,00 | 121 | 48,40 | 0,000010 |
| No | 77 | 44,00 | 52 | 75,36 | - | - | 129 | 51,60 | |
| RVS | |||||||||
| Yes | 169 | 96,57 | 68 | 98,55 | 6 | 100,00 | 243 | 97,20 | 0,683030 |
| No | 6 | 3,43 | 1 | 1,45 | - | - | 7 | 2,80 | |
| Adverse events | |||||||||
| Yes | 38 | 21,71 | 15 | 21,74 | 1 | 16,67 | 54 | 21,60 | 0,996618 |
| No | 137 | 78,29 | 54 | 78,26 | 5 | 83,33 | 196 | 78,40 | |
| City | |||||||||
| Belém | 173 | 69,20 | |||||||
| Other | 77 | 30,80 | |||||||
SOF: sofosbuvir; DAC: daclatasvir; RBV: ribavirin; SMV: simeprevir; NA: does not apply; SVR: sustained virological response. *Comparison between SOF + DAC ± RBV and SOF + SMV ± RBV. Conventional signal used: - Numerical data equal to zero, not resulting from rounding.
Most patients who received treatment with sofosbuvir and daclatasvir had genotype 1 infection (66.29%) and genotype 3 (32.00%); 102 (58.29%) were carriers of liver cirrhosis; and 74 (42.29%) have tried other treatments previously. Information on the total SVR rate and categorized by some clinical characteristics of this group of patients can be found in Figure 1. The total SVR rate found in this subgroup was 96.57%. This regimen showed a slight superiority in the SVR rate for patients with genotype 1 (98.27%), compared to patients with genotype 3 (92.85%); however, this difference showed no statistical relevance (p > 0.05). All patients with genotype 2 presented SVR. The frequency of adverse effects was 21.71%, the main ones shown in Figure 2.
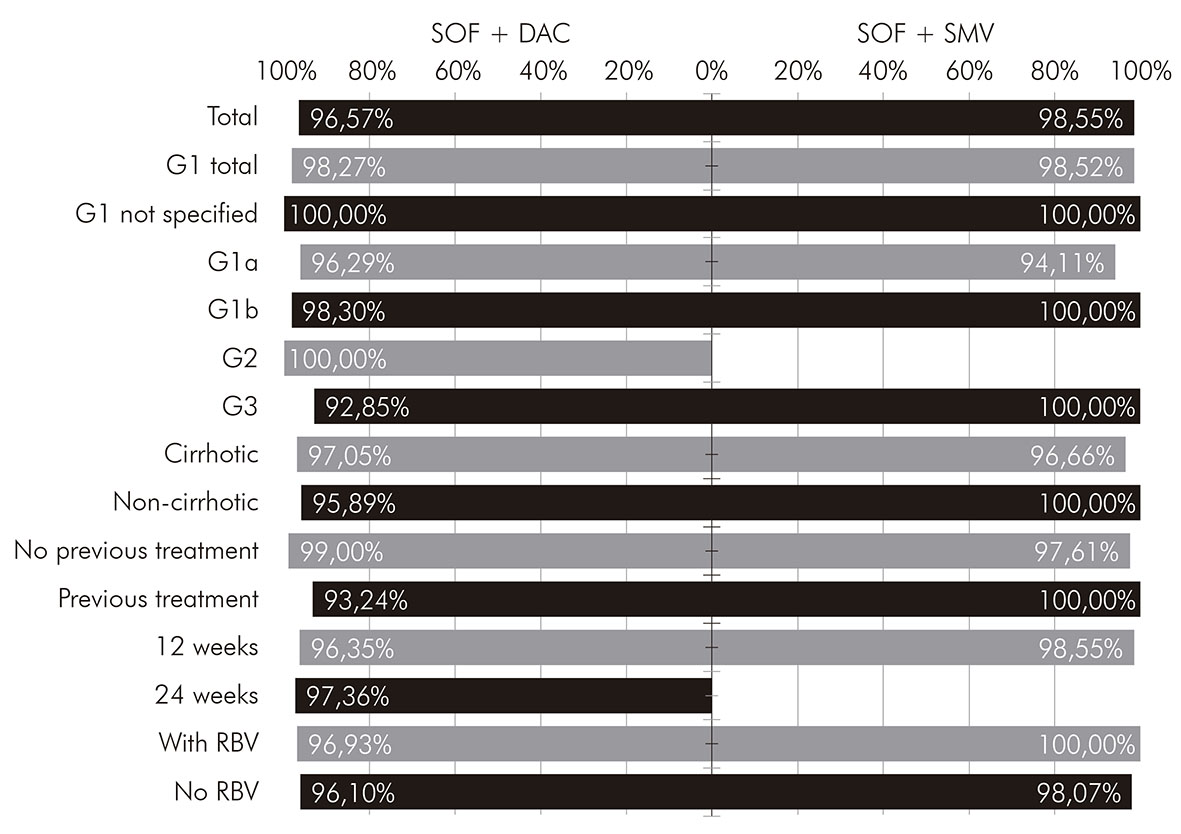
* Total and according to the clinical characteristics of the patients. SOF: sofosbuvir; DAC: daclatasvir; SMV: simeprevir; G1: genotype 1; G2: genotype 2; G3: genotype 3; RBV: ribavirin.
Figure 1 - SVR rates, by treatment regimen, of patients with chronic hepatitis C treated with direct-action antiviral agents at FSCMPA in Belém, Pará State, Brazil, between May 2017 and March 2019*
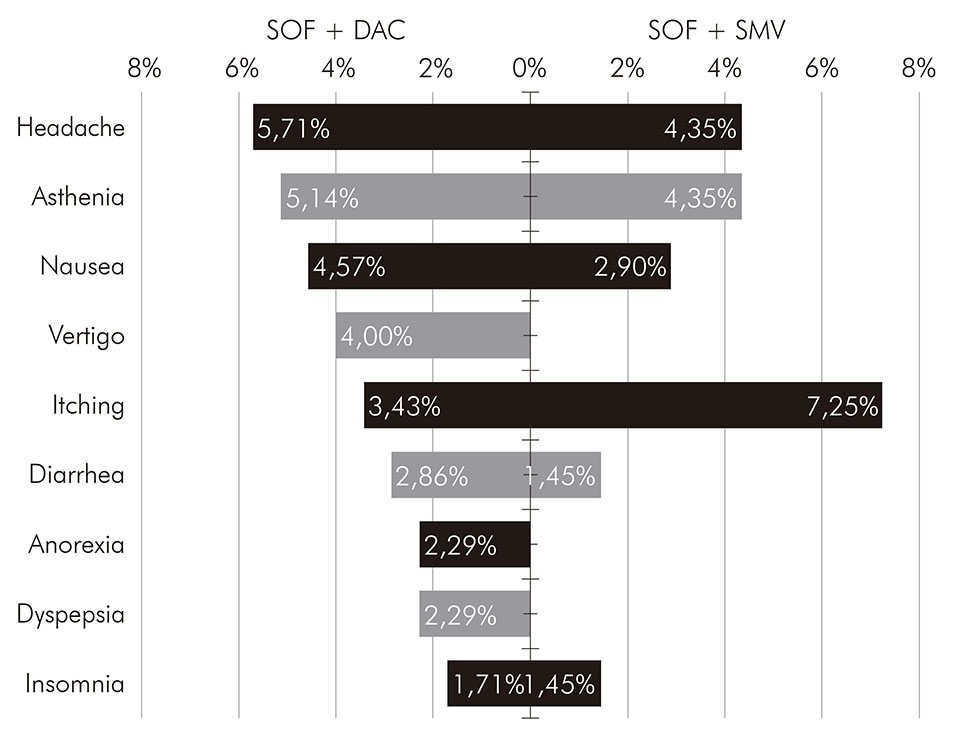
SOF: sofosbuvir; DAC: daclatasvir; SMV: simeprevir.
Figure 2 - Frequency of adverse events, by treatment regimen, of patients with chronic hepatitis C treated with direct-action antiviral agents at FSCMPA in Belém, Pará State, Brazil, between May 2017 and March 2019
Patients who received treatment with sofosbuvir and simeprevir were infected almost entirely by genotype 1 (98.55%), with only one patient infected with genotype 3 and none with genotype 2; 30 (43.48%) were carriers of liver cirrhosis; and 27 (39.13%) have experienced some treatment previously. Information on the total SVR rate and categorized by clinical characteristics of this group of patients can be found in Figure 1. The total SVR rate found in this subgroup was 98.55%, slightly higher than the rate for those who received sofosbuvir and daclatasvir; however, without statistical significance (p > 0.05). The frequency of adverse effects in this regimen was 21.74%, very similar to that found in the group that received sofosbuvir and daclatasvir. The most frequent were headache, asthenia and itching, as shown in Figure 2.
A total of six patients carried out alternative regimens, five of them with sofosbuvir and ribavirin, all infected by genotype 2, and one with sofosbuvir, pegylated interferon and ribavirin, infected by genotype 3. All of them had SVR. Clinical information on this subgroup can be found in Table 2.
Table 2 - Sustained virological response rates and clinical characteristics of patients who received alternative regimens for chronic hepatitis C at FSCMPA in Belém, Pará State, Brazil, from May 2017 to March 2019
| Proposed scheme | SOF+INF+RBV | SOF+RBV |
|---|---|---|
| Number of patients | 1 | 5 |
| Duration in weeks | 12 (100,00%) | 12 (100,00%) |
| Genotype | 3 (100,00%) | 2 (100,00%) |
| Cirrhosis (%) | 100,00% | 80,00% |
| Previous treatment (%) | 100,00% | - |
| RVS (%) | 100,00% | 100,00% |
SOF: sofosbuvir; INF: interferon alpha; RBV: ribavirin; SVR: sustained virological response. Conventional signal used: - Numerical data equal to zero, not resulting from rounding.
In this study, seven non-responder patients were found, most of them women (71.43%), with a mean age of 62 years, infected by genotype 3 (57.14%), patients with cirrhosis (57.14%), with treatment lasting 12 weeks (85.71%). The SVR rate in genotype 3 was the lowest among all of them (93.10%). Other clinical and demographic characteristics about these patients are shown in Chart 1 and Table 3.
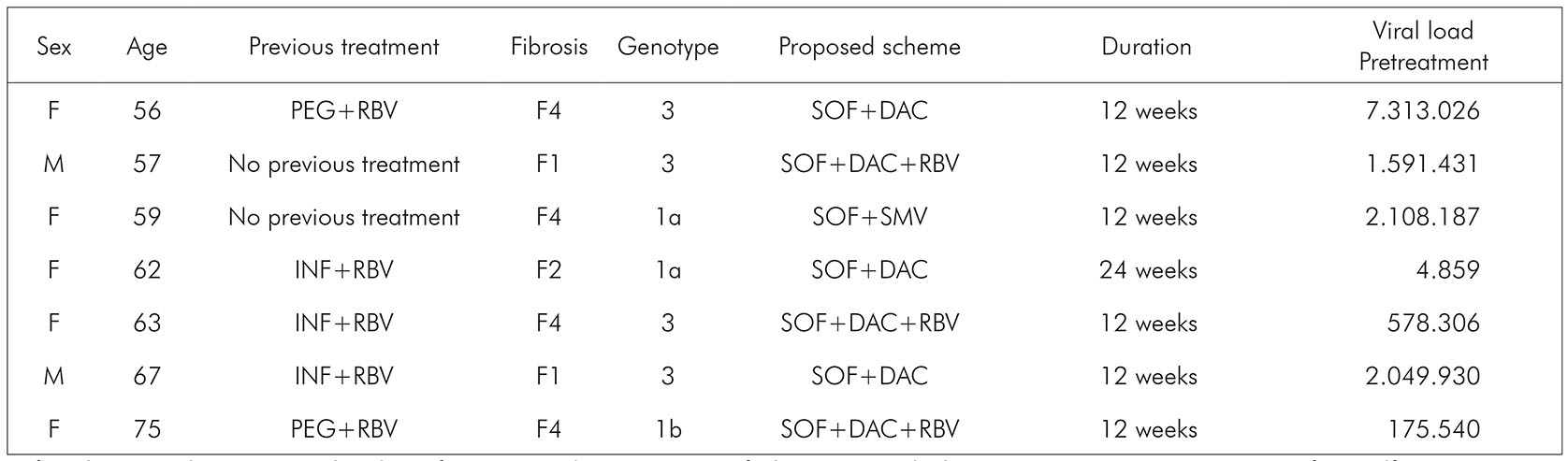
F: female; M: male; PEG: pegylated interferon; RBV: ribavirin; SOF: sofosbuvir; DAC: daclatasvir; SMV: simeprevir; IFN: interferon alfa.
Chart 1 - Clinical and demographic characteristics of non-responder patients to direct-acting antiviral agents at FSCMPA in Belém, Pará State, Brazil, from May 2017 to March 2019
Table 3 - Clinical and demographic characteristics of non-responder patients to direct-acting antiviral agents at the Fundação Santa Casa de Misericórdia in Belém, Pará, from May 2017 to March 2019, by treatment scheme
| Variables | SOF + DAC ± RBV | SOF + SMV ± RBV | Total | |||
|---|---|---|---|---|---|---|
| Age | Average | Min.-max. | Average | Min.-max. | Average | Min.-max. |
| 63,33 | 56-75 | 59 | 59 | 62,71 | 56-75 | |
| N = 6 | % | N = 1 | % | N = 7 | % | |
| Sex | ||||||
| Male | 2 | 33,33 | - | - | 2 | 28,57 |
| Female | 4 | 66,67 | 1 | 100,00 | 5 | 71,43 |
| Genotype | ||||||
| 1 total | 2 | 33,33 | 1 | 100,00 | 3 | 42,86 |
| 1a | 1 | 50,00 | 1 | 100,00 | 2 | 66,67 |
| 1b | 1 | 50,00 | - | - | 1 | 33,33 |
| 2 | - | - | - | - | - | - |
| 3 | 4 | 66,67 | - | - | 4 | 57,14 |
| Viral load (UI/mL) | ||||||
| < 800.000 | 3 | 50,00 | - | - | 3 | 42,86 |
| ≥ 800.000 | 3 | 50,00 | 1 | 100,00 | 4 | 57,14 |
| Previous treatment | ||||||
| Yes | 5 | 83,33 | - | - | 5 | 71,43 |
| No | 1 | 16,67 | 1 | 100,00 | 2 | 28,57 |
| Fibrosis | ||||||
| F0-F3 | 3 | 50,00 | - | - | 3 | 42,86 |
| F4 | 3 | 50,00 | 1 | 100,00 | 4 | 57,14 |
| Duration of treatment | ||||||
| 12 weeks | 5 | 83,33 | 1 | 100,00 | 6 | 85,71 |
| 24 weeks | 1 | 16,66 | - | - | 1 | 14,29 |
| Addition of RBV | ||||||
| Yes | 3 | 50,00 | - | - | 3 | 42,86 |
| No | 3 | 50,00 | 1 | 100,00 | 4 | 57,14 |
SOF: sofosbuvir; DAC: daclatasvir; RBV: ribavirin; SMV: simeprevir. Conventional signal used: - Numerical data equal to zero, not resulting from rounding.
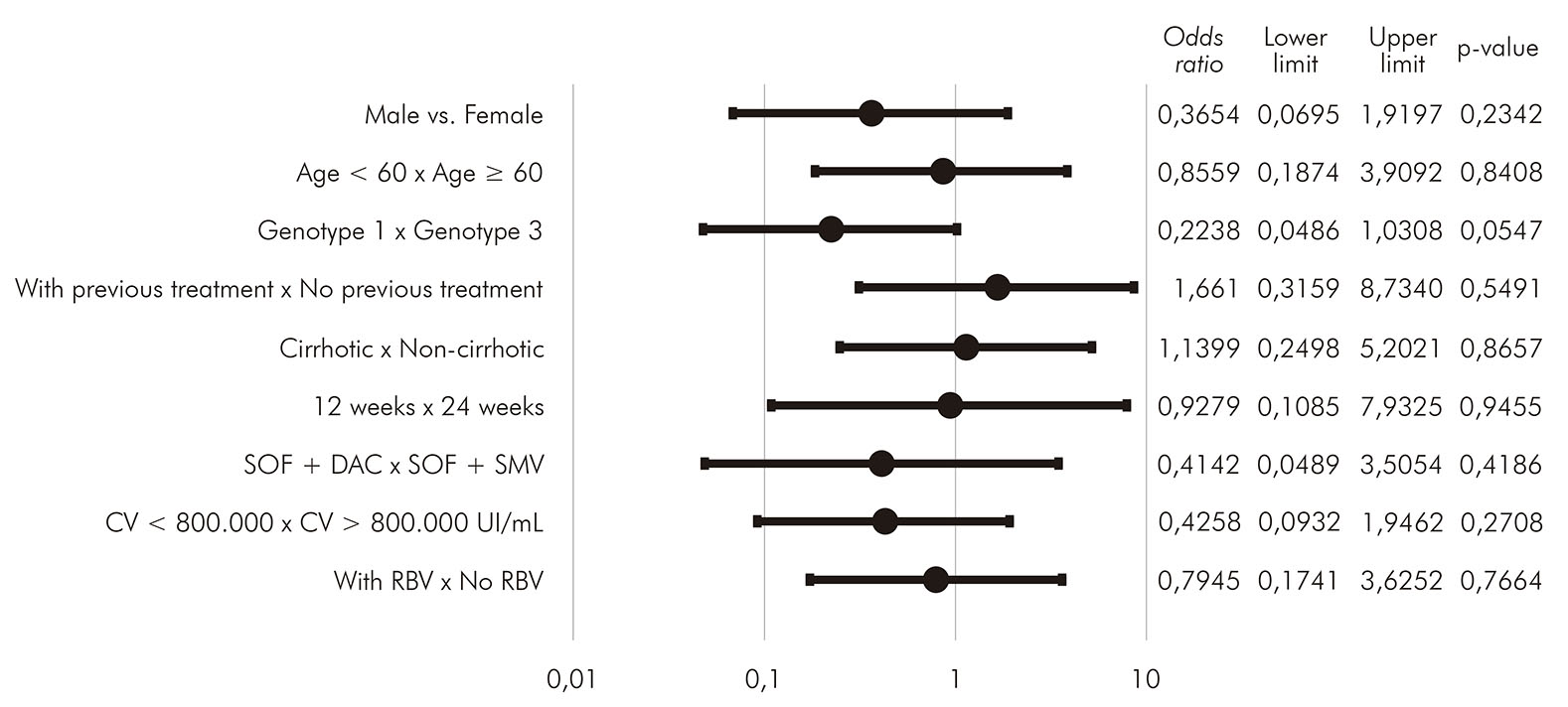
Statistical analyses were carried out in order to search for possible associations between clinical characteristics and the existence of SVR. The variables analyzed were gender, age greater than or less than 60 years, genotype, existence of previous treatment, cirrhosis, treatment time between 12 and 24 weeks, treatment regimen with sofosbuvir and daclatasvir or sofosbuvir and simeprevir, viral load greater than or less than 800,000 IU/mL and addition of ribavirin. No association showed statistical significance (p > 0.05). Details about these analyses can be seen in Figure 3.
DISCUSSION
Second-generation direct-acting antivirals agents demonstrated high effectiveness in the population undergoing treatment at FSCMPA, with a general SVR rate of 97.20%. This result is in agreement with international studies, such as COSMOS11 and OPTIMIST-112, and also those conducted at national level and in several regions of Brazil. In the Southern Region, studies conducted by Ferreira et al.13, Holzmann et al.14 and Molinar et al.15 found SVR rates of 91.60%, 93.40% and 94.20% in the states of Paraná, Rio Grande do Sul and Santa Catarina, respectively. In the Northeast Region, Rolim et al.16 found a percentage of 95% in the city of Fortaleza, state of Ceará. In the Southeast Region, Azevedo17 found a percentage of 96.30% in Rio de Janeiro city. A nationwide study conducted by Cheinquer et al.18 in which patients from several cities were evaluated, showed the same result, with SVR rates ranging from 88% to 97%, depending on the genotype of the virus. All these data confirm the effectiveness of these drugs.
The studied population also showed clinical and demographic characteristics similar to those of the populations analyzed in other studies, such as the national studies mentioned before13,14,15,16,17,17 showing a predominantly male, elderly, urban population, with a high percentage of cirrhotic patients and patients with previous treatments.
With regard to genotype, the highest prevalence corresponded to genotype 1 (73.60%), followed by genotype 3 (23.20%), according to the prevalence of these genotypes at national level19. Genotype 3 infection has been considered difficult to treat, especially when associated with cirrhosis, with lower rates of SVR, showed in international studies such as FISSION20, POSITRON21 and ALLY-322. In the present analysis, four of the seven non-responder patients were infected with genotype 3, and only two of these patients were cirrhotic. The SVR rate in genotype 3 was the lowest among all (93.10%). However, the small number of patients in this study makes it difficult to be analyzed.
It is important to notice that most patients (54.00%) were carriers of liver cirrhosis. Possibly, these findings are associated with the difficulty in diagnosing hepatitis C in the early stages due to its mostly asymptomatic feature, and also due to the low efficacy and tolerability of interferon treatments previously used. In the present sample, 40.80% of the patients had tried, without success, any of these treatment modalities.
The main adverse events found in patients receiving sofosbuvir and daclatasvir were headache (5.71%), asthenia (5.14%) and nausea (4.57%). A similar result was found in the studies conducted by Sulkowski et al.23 and Medeiros et al.24 with patients who received the same treatment regimen, and the same adverse events mentioned were more frequently demonstrated. In patients who received sofosbuvir and simeprevir, the most frequent adverse effect was pruritus (7.25%), followed by the other ones already mentioned. These results confirm the good tolerability of direct-acting antivirals and low frequency of adverse events.
Regarding the addition of ribavirin to the therapeutic regimen, this did not seem to be an important factor in the range of SVR, with little proportional difference in SVR rates in both group that received sofosbuvir and daclatasvir (96.93% vs.96.10%) as well as in what received sofosbuvir and simeprevir (100.00% vs. 98.07%).
It is important to note that, among the patients who received sofosbuvir and simeprevir, one of them was infected by genotype 3, and for this genotype, according to the PCDT 2017 (at that time of the beginning of the research) simeprevir was not recommended, demonstrating possible failure in care. Still, the patient presented SVR without any problems. In addition, since February 2019, simeprevir has ceased to be recommended by the PCDT of the Ministry of Health9 and has been excluded from the pharmacological arsenal of the SUS due to the introduction of new therapeutic alternatives of greater effectiveness, such asand velpatasvir/sofosbuvir, for example, which are considered pangenotypic regimens because they treat all HCV genotypes, while simeprevir was indicated only for monoinfected patients with HCV genotype 1, without cirrhosis or with Child-A cirrhosis25.
Chronic hepatitis C still continues to be one of the main causes of cirrhosis and liver transplantation in adults in most countries26,27. Thus, WHO has adopted the aim of eliminating HCV until 203028. It is important to focus on disease prevention as well as early diagnosis and treatment, avoiding complications resulting from chronic HCV infection. The adoption of direct-acting antivirals agents in Brazil could corroborate the goal of eliminating the virus, as well as increase the number of patients treated by non-specialists, thus expanding access to treatment29.
The limitations of the present study may be some selection bias due to the impossibility of evaluating all patients treated with direct-acting antivirals during the data collection period, due to the lack of clinical follow-up information of many patients, who had to be excluded from the final sample, making it impossible to identify the number of responder and non-responder patients to the new treatment.
CONCLUSION
The results of this observational study, in a sample of patients chronically infected with HCV in the Pará State, showed that of direct-acting antivirals of second generation (daclatasvir, sofosbuvir and simeprevir) presented a high rate of SVR, reflecting excellent effectiveness and tolerability according to previous studies conducted in other countries and other regions of Brazil.
REFERENCES
1 World Health Organization. Hepatitis C: fact sheets. Geneva: World Health Organization; 2019. [ Links ]
2 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Hepatites virais 2019. Bol Epidemiol. 2019 jul;50(17):1-71. [ Links ]
3 Li HC, Lo SY. Hepatitis C virus: virology, diagnosis and treatment. World J Hepatol. 2015 Jun;7(10):1377-89. [ Links ]
4 Coelho ME. Progressos terapêuticos na hepatite C [dissertação]. Porto (PT): Universidade do Porto, Faculdade de Medicina; 2015. [ Links ]
5 Mello CEB. Tratamento da hepatite crônica pelo vírus C: novas perspectivas. J Bras Med. 2014 jan-fev;102(1):23-32. [ Links ]
6 Lanini S, Mammone A, Puro V, Girardi E, Bruzzi P, Ippolito G. Triple therapy for hepatitis C improves viral response but also increases the risk of severe infections and anaemia: a frequentist meta-analysis approach. New Microbiol. 2014 Jul;37(3):263-76. [ Links ]
7 Islam MMSU, Sarker MN, Rahman M, Uddin MR, Rahman ATMA, Biswas G. Management of hepatitis C virus infection - new era has been started. Faridpur Med Coll J. 2014 Jul;9(2):92-7. [ Links ]
8 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância, Prevenção e Controle das Infecções Sexualmente Transmissíveis, do HIV/Aids e das Hepatites Virais. Protocolo clínico e diretrizes terapêuticas para hepatite C e coinfecções. Brasília: Ministério da Saúde; 2015. [ Links ]
9 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de DST, Aids e Hepatites Virais. Protocolo clínico e diretrizes terapêuticas para hepatite C e coinfecções. Brasília: Ministério da Saúde ; 2019. [ Links ]
10 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de DST, Aids e Hepatites Virais. Protocolo clínico e diretrizes terapêuticas para hepatite C e coinfecções. Brasília: Ministério da Saúde ; 2017. [ Links ]
11 Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014 Nov;384(9956):1756-65. [ Links ]
12 Kwo P, Gitlin N, Nahass R, Bernstein D, Etzkorn K, Rojter S, et al. Simeprevir plus sofosbuvir (12 and 8 weeks) in hepatitis C virus genotype 1-infected patients without cirrhosis: OPTIMIST-1, a phase 3, randomized study. Hepatology. 2016 Aug;64(2):370-80. [ Links ]
13 Ferreira VL, Borba HHL, Wiens A, Pedroso MLA, Radunz VFC, Ivantes CAP, et al. Effectiveness and tolerability of direct-acting antivirals for chronic hepatitis C patients in a Southern state of Brazil. Braz J Infect Dis. 2018 May-Jun;22(3):186-92. [ Links ]
14 Holzmann I, Tovo CV, Minmé R, Leal MP, Kliemann MP, Ubirajara C, et al. Effectiveness of chronic hepatitis C treatment with direct-acting antivirals in the Public Health System in Brazil. Braz J Infect Dis. 2018 Jul-Aug;22(4):317-22. [ Links ]
15 Molinar E, Oliveira JV, Biff MM, Bez PR. Perfil epidemiológico e resposta virológica sustentada de pacientes com hepatite C crônica em resposta ao tratamento com os novos antivirais de ação direta em dois serviços de referência do extremo sul catarinense. Arq Catarin Med. 2019 jan-mar;48(1):10-21. [ Links ]
16 Rolim FE, Braga LLBC, Lima JMC, Mello FSF, Pinho CS, Hyppolito EB. Hepatite crônica pelo vírus C: avaliação da resposta virológica ao tratamento com os novos antivirais de ação direta. Rev Med UFC. 2018 dez;58(4):8-12. [ Links ]
17 Azevedo DAF. Tratamento de hepatite C crônica com drogas antivirais de ação direta de segunda geração: Sofosbuvir, Simeprevir, Daclatasvir - resposta virológica sustentada no ambulatório de doenças do fígado do Hospital Universitário Gaffrée e Guinle [dissertação]. Rio de Janeiro (RJ): Universidade Federal do Estado do Rio de Janeiro; 2018. [ Links ]
18 Cheinquer H, Coelho HS, Aires RS, Quintela ED, Lobato C, Medeiros Filho JE, et al. New direct action antivirals containing regimes to treat patients with hepatitis C chronic infection: first results from a national real-world registry of the Brazilian Hepatology Society. J Hepatol. 2017;66(1 Suppl): S508. [ Links ]
19 Campiotto S, Pinho JRR, Carrilho FJ, Da Silva LC, Souto FJD, Spinelli V, et al. Geographic distribution of hepatitis C virus genotypes in Brazil. Braz J Med Biol Res. 2005 Jan;8(1):41-9. [ Links ]
20 Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013 May;368(20):1878-87. [ Links ]
21 Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013 May;368(20):1867-77. [ Links ]
22 Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015 Jan;61(4):1127-35. [ Links ]
23 Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014 Jan;370(3):211-21. [ Links ]
24 Medeiros T, Salviato CM, do Rosário NF, Saraiva GDN, Esberard EBC, Almeida JR, et al. Adverse effects of direct acting antiviral-based regimens in chronic hepatitis C patients: a Brazilian experience. Int J Clin Pharm. 2017 Oct;39(6):1304-11. [ Links ]
25 Ministério da Saúde (BR). Secretaria de Ciência, Tecnologia e Insumos Estratégicos. Comissão Nacional de Incorporação de Tecnologias no SUS. Simeprevir para o tratamento da hepatite C. Brasília: Ministério da Saúde ; 2019. Relatório de recomendação, n.º 428. [ Links ]
26 World Health Organization. Global health sector strategy on viral hepatitis 2016-2021 [Internet]. Genebra: World Health Organization; 2016 [cited 2017 Mar 10]. Available from: Available from: https://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/ . [ Links ]
27 World Health Organization. Global hepatitis report, 2017 [Internet]. Genebra: World Health Organization ; 2017 [cited 2017 Mar 10]. Available from: Available from: https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/ . [ Links ]
28 World Health Organization. Combating hepatitis B and C to reach elimination by 2030 [Internet]. Genebra: World Health Organization ; 2016 [cited 2016 Dez 11]. Available from: Available from: https://www.who.int/hepatitis/publications/hep-elimination-by-2030-brief/en/ . [ Links ]
29 Cohn J, Roberts T, Amorosa V, Lemoine M, Hill A. Simplified diagnostic monitoring for hepatitis C, in the new era of direct-acting antiviral treatment. Curr Opin HIV AIDS. 2015 Sep;10(5):369-73. [ Links ]
How to cite this article / Como citar este artigo: Borges Neto FC, Souza MGL, Novaes ICRB, Silveira VS, Nunes MP, Miranda GCBM, et al. Effectiveness of treatment with direct-acting antiviral drugs in patients with hepatitis C treated at a referral center in the Pará Sate, Brazil, from 2017 to 2019. Rev Pan Amaz Saude. 2020;11:e202000468. Doi: http://dx.doi.org/10.5123/S2176-6223202000468
Received: August 23, 2019; Accepted: June 08, 2020











 texto en
texto en