Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Pan-Amazônica de Saúde
Print version ISSN 2176-6215On-line version ISSN 2176-6223
Rev Pan-Amaz Saude vol.12 Ananindeua 2021 Epub Jan 13, 2021
http://dx.doi.org/10.5123/s2176-6223202000476
ORIGINAL ARTICLE
Seropositivity and risk factors associated with Toxoplasma gondii infection in patients treated at the Municipal Laboratory of Oriximiná, Pará State, Brazil
1Universidade Federal Fluminense, Instituto Biomédico, Departamento de Microbiologia e Parasitologia, Niterói, Rio de Janeiro, Brasil
2Fundação Oswaldo Cruz, Instituto Oswaldo Cruz, Laboratório de Toxoplasmose e Outras Protozooses, Rio de Janeiro, Rio de Janeiro, Brasil
3Laboratório Municipal de Oriximiná, Oriximiná, Pará, Brasil
4Universidade Federal Fluminense, Instituto de Matemática e Estatística, Departamento de Estatística, Niterói, Rio de Janeiro, Brasil
OBJECTIVES:
Evaluate the frequency of anti-Toxoplasma gondii antibodies and the risk factors inherent to infection with this parasite and to compare serological diagnostic techniques in patients treated at the Municipal Laboratory of Oriximiná, Pará State, Brazil.
MATERIALS AND METHODS:
Serum samples were collected from patients, as well as socioeconomic and environmental data by form application. These samples were tested for IgM and IgG antibodies by indirect enzyme-linked immunosorbent assay (ELISA) and indirect fluorescent antibody test (IFAT).
RESULTS:
A total of 521 samples were collected. The frequency of seropositive individuals with T. gondii was 68.7%. In 51%, only IgG antibodies were found, while in 17.7% IgG/IgM antibodies were detected, a profile compatible with acute infection. Almost perfect agreement between ELISA and IFAT tests was found regarding the IgG (Kappa = 0.84). In univariate analysis, the variables significantly associated with positivity for T. gondii were: age group, consumption of greens and vegetables, previous positive result, abortion, and presence of cat in the house. As for the logistic regression, it was identified that a higher age group, the presence of cats as pets, and a lower income range were factors that presented a higher risk of infection by T. gondii.
CONCLUSION:
The high frequency of T. gondii seropositive patients seen at the Municipal Laboratory of Oriximiná was evidenced, as well as a lack of perfect agreement between IFAT and ELISA, demonstrating the need to use more than one laboratory technique for the detection of anti-T. gondii antibodies.
Keywords: Toxoplasmosis; Diagnosis; Serology; Risk Factor.
INTRODUCTION
Toxoplasmosis is a zoonosis caused by Toxoplasma gondii, a protozoan that can infect different species of birds and mammals. The transmission to humans occurs through the ingestion of raw or undercooked meat containing cysts or through the ingestion of water and food, such as contaminated vegetables, with oocysts that are released in the feces of infected cats, the definitive hosts of this parasite. In addition, primary infection in pregnant women can cause congenital transmission1,2.
In immunocompetent patients, the infection by T. gondii is usually asymptomatic. In symptomatic cases, in the acute phase of infection, symptoms are generally nonspecific, including lymphadenopathy, headache and fever1. In the chronic phase, retinochoroiditis can occur, which can progress to vision loss3. In congenital toxoplasmosis, infection can lead to abortion, fetal death, premature birth or eye damage4. In immunocompromised individuals, among whom are transplant patients or carriers of the human immunodeficiency virus, the infection can progress to encephalitis, myocarditis and eye lesions2,5.
Human toxoplasmosis has a worldwide distribution, and its prevalence is related to the country of occurrence6. A high prevalence of T. gondii infection is observed in tropical countries with hot and humid climates2. In Brazil, there are several studies on seroprevalence of toxoplasmosis. The frequency indexes of anti-T. gondii antibodies in studies carried out in a population of different age groups ranged from 21.5% in Natal, Rio Grande do Norte State, to 97.4% in Jauru, Mato Grosso State7. However, research on this parasite is still scarce in some regions of the country, especially in Amazon cities6.
Studies conducted in Pará State highlighted high prevalence, reaching 81.9%, as reported in Novo Repartimento city, in addition to outbreaks of acute symptomatic infections reported in Almeirim and Ponta de Pedras8,9,10. In general, the main research efforts in the Amazonian states have focused on two aspects: urban areas and indigenous populations11. However, in some cities of the state, such as Oriximiná, epidemiological information on toxoplasmosis is scarce.
Thus, the objectives of the present study were to identify the frequency of anti-T. gondii antibodies in serum from patients treated at the Municipal Laboratory of Oriximiná and the risk factors associated with infection by the parasite; to compare the results obtained between the serological techniques used for the diagnosis of anti-T. gondii antibodies; and to identify which technique would be the most appropriate for serological diagnosis of this protozoan.
MATERIALS AND METHODS
ETHICAL ASPECTS
This study was approved by the Research Ethics Committee of the Universidade Federal Fluminense (UFF), under document No. 3,152,430, CAAE 03218818.80000.5243, in January 2019, and Instituto Oswaldo Cruz, CAEE 03218818.8.3001.5248, in February 2019.
STUDY AREA
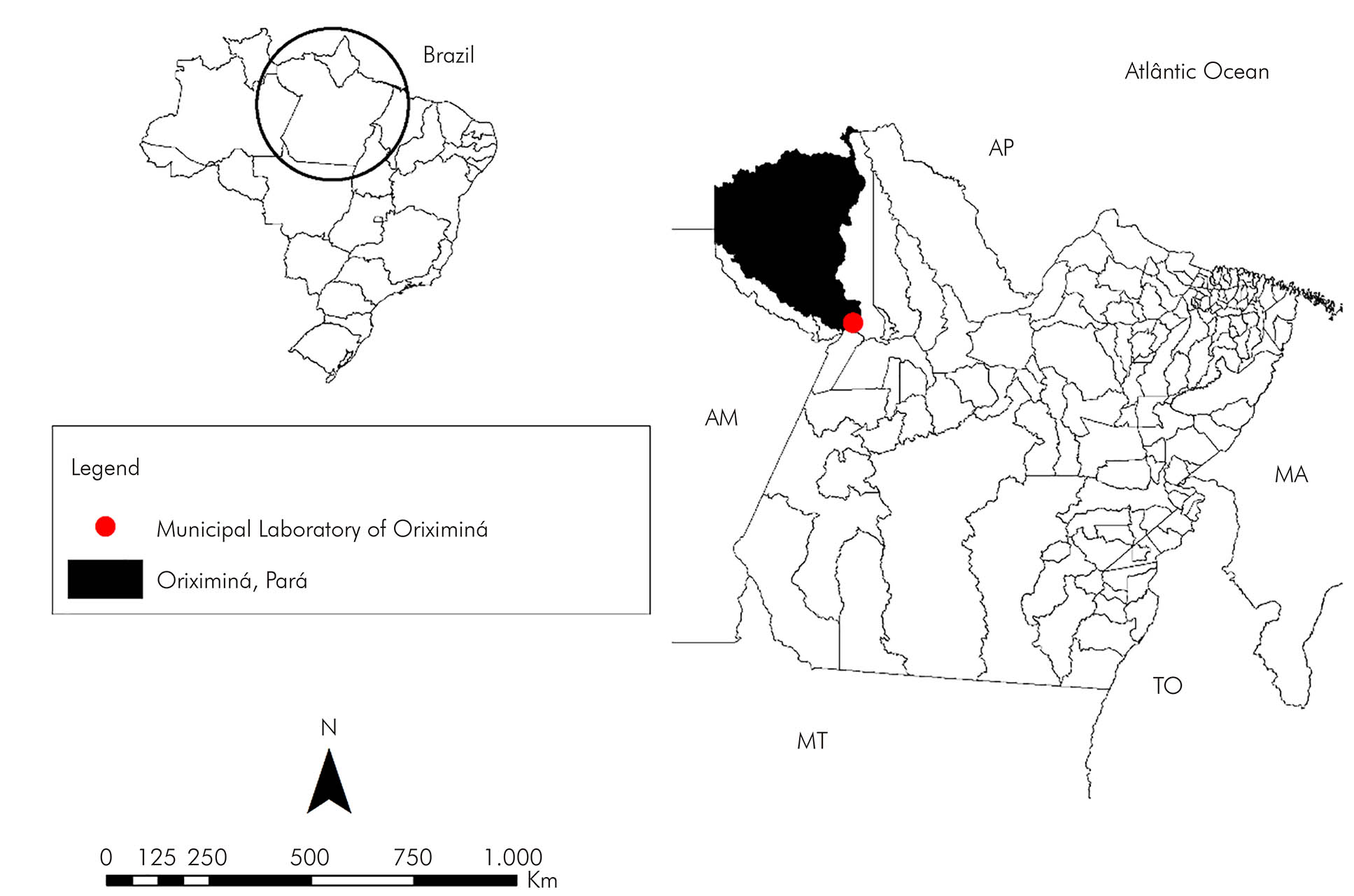
The Oriximiná city is inserted in the Mesoregion of Baixo Amazonas, being the second largest in an area of Pará State, with approximately 107,603.2 km2 (Figure 1). In addition to bordering other municipalities in the state, it also borders Roraima and Amazonas States and the of Guyana and Suriname countries. The city contains large rivers, among which the Trombetas and Amazonas rivers stand out12. The climate of the region is hot and humid and, in the less rainy period, the temperature varies between 35 and 37 ºC, between June and August. From December to July, the rainy season, the temperature is around 32 ºC13. Oriximiná has a total population of 62,963 inhabitants14. Ethnically, this population has an Indigenous, African, and European origin. In the main urban settlement in the city, two public hospitals, five basic health units, a private hospital and a municipal laboratory serve the entire population. Patients who were seen at this laboratory were invited to participate in this study.
SAMPLING
In February 2019, serum samples were collected from 521 patients treated at the Municipal Laboratory of Oriximiná. People in the waiting room were invited to participate in the study, being of any age and gender, as well as pregnant women. Those who agreed to participate signed the informed consent or informed assent form. In the case of people under 18 years old, a legal guardian signed a declaration of free and informed consent. Indigenous people were not included, as the technical team did not have the scientific or linguistic capacity to work with them.
After joining the study, participants were invited to fill out a semi-structured form containing socioeconomic and environmental data, and were subsequently referred for blood collection. Blood samples were collected in tubes without anticoagulants, containing clot-activating gel. Then, they were centrifuged at 2,500 rpm for 5 min. Microtubes containing aliquots of serum were identified, stored at -20 ºC and, later, sent to the Laboratory of Toxoplasmosis and Other Protozooses, Fundação Oswaldo Cruz (Fiocruz), in Rio de Janeiro, in specific transport boxes for serological tests.
SEROLOGICAL TESTS
In the Laboratory of Toxoplasmosis and other Protozooses, the samples were subjected to the search for IgM and IgG anti-T. gondii antibodies using the indirect enzyme immunoassay (ELISA) (InterKit TOXO Katal®, Belo Horizonte, Brazil) and the indirect fluorescent antibody test (IFAT), according to the protocol described by Camargo15. The indirect ELISA was performed following the technical recommendations of the kit manufacturer. In ELISA plate, previously sensitized with the T. gondii antigen, were added negative control - calibration solutions used to calculate the cut-off point - and positive control. Then, the diluent and serum samples from each patient were added to the wells. Subsequently, the plate was put in the incubator for 30 min at 37 ºC. At the end of the incubation, the plate was washed with a previously prepared washing solution to remove those antibodies that did not adsorb to the antigen, and then the conjugate (human anti-immunoglobulin conjugated to the enzyme) was added. After the incubation, the plate was once again washed, and substrate solutions were added to it. A blocking solution was added to complete the reaction, and the plate was read in an automatic microplate reader (Biochrom EZ Read 400) with 450 nm wavelength and 650 nm differential. The reaction was defined according to the cut-off value.
For the IgM search on IFAT, 1:16 and 1:64 dilutions were made; and, for IgG screening, the dilutions were of 1:16, 1:64, 1:256, 1:1024 and 1:4096. The positive and negative controls were previously analyzed using samples stored in the laboratory freezers. In addition, anti-human immunoglobulin IgG and anti-human immunoglobulin IgM conjugates (Sigma®, St. Louis, USA) were also used. The reactions that occurred in serum dilutions ≥ 1:16, with complete fluorescence in the parasite membrane and in at least 50% of the tachyzoites observed under the microscope, were considered positive. The slides were read under an epifluorescence microscope (Nikon E400) Y-FL, with a 400x magnification.
Patients who were IgM positive and IgG negative on IFAT for T. gondii also had their serum subjected to rheumatoid factor testing with the Immuno-LATEX kit (Wama Diagnóstica®, São Carlos, Brazil), in order to exclude the possibility of false positives, that is, a cross reaction between antibodies produced to another infectious agent that can adsorb to the T. gondii antigen. For the qualitative test, the kit manufacturer's recommendation was followed, with positive control serum in the first well, negative control serum in the second well and samples in the remaining wells. Then, the latex suspension was added to each well. Each circular area containing the serum and the latex suspension was homogenized. Performing smooth rotation movements and under a light source, the possible formation of agglutination was observed for 2 min, as occurs in the positive control. The formation of agglutination indicates that the sample is positive for rheumatoid factor.
STATISTICAL ANALYSIS
Comparisons of the results obtained by serological techniques were performed using the McNemar test and the Kappa coefficient. The Kappa index was interpreted, as described by Landis and Koch16, as follows: Kappa < 0 does not indicate agreement; between 0 and 0.20, minimum agreement; 0.21 to 0.40, reasonable agreement; from 0.41 to 0.60, moderate agreement; from 0.61 to 0.80, substantial agreement; and from 0.81 to 1.0, almost perfect agreement. The McNemar test was used to verify whether the disagreements between the techniques could be considered a random fluctuation. The hypothesis of agreement between the techniques would be accepted when the calculated p-value was greater than the level of significance adopted (5%).
Bivariate analyzes, to verify the association between positivity for T. gondii and its possible risk factors, were performed using Fisher's exact test. The following dependent variables were used: age group; gender; number of residents in the same household; family income; water source; water treatment; consumption of fruits and vegetables; hygiene of fruits and vegetables; meat consumption; pork consumption; consumption of wild animal meat; constant contact with the soil in its activities; prior knowledge about toxoplasmosis; previous results of tests for toxoplasmosis; having suffered an abortion, in the case of women; and having a cat as a pet. The risk factors considered minimally significant (which presented p ≤ 0.25, according to Hosmer et al.17) were evaluated as independent variables as to their ability to jointly model the chance of finding a positive result for T. gondii, through the construction of a logistic regression model, using a stepwise procedure with conditional inputs and levels of input significance (of variable in the model) equal to 0.20 and permanence (of variable in the model) equal to 0.15 (according to Lee and Koval18). Then, the p-values of the significance test of the respective coefficients and the odds ratio (OR) values with a 95% confidence interval (CI) were presented. All analyses were performed using the SPSS v18.0 software (SPSS Inc., Chicago, USA).
RESULTS
According to the information recovered from the 521 forms, most participants were adults (285) and children (107). The age group in this study varied from 2 months to 90 years, with an average of 31 years old. Individuals of both sexes participated in the study, with the majority being female (331). Most participants lived in houses with four to five residents, followed by six or more residents. It was also found that 350 participants had an income up to a minimum wage (R$ 938.00, value at the time of the study); 243 received municipal water in their houses; and 314 said they treated the water with a 1% hypochlorite solution by themselves. Most people reported consuming greens and vegetables (418), meat (507), including pork (298), and wild animals (296). More than half participants said that they did not work/deal/play with soil (278) nor have a cat as a pet (351). Moreover, 290 people never heard of toxoplasmosis and 454 had not being tested for toxoplasmosis. Among the women interviewed, 83 reported having already aborted (Table 1).
Table 1 - Risk factors associated with T. gondii infection in serum samples from patients treated at the Municipal Laboratory of Oriximiná, Pará State, Brazil, verified by bivariate analysis
| Variable | Total of samples | Positive | OR (IC 95%) | p-value | |
|---|---|---|---|---|---|
| N | % | ||||
| Age group | |||||
| Elderly (≥ 60 years old) | ”57 | 54 | 94,7 | - | < 0,001* |
| Adult (19-59 years old) | 285 | 232 | 81,4 | ||
| Teenager (12-18 years old) | 72 | 39 | 54,2 | ||
| Child (≤ 11 years old) | 107 | 33 | 30,8 | ||
| Gender | |||||
| Male | 190 | 127 | 66,8 | 0,873 (0,595; 1,279) | 0,493 |
| Female | 331 | 231 | 69,8 | ||
| Number of residents in the household | |||||
| 6 or more | 144 | 97 | 67,4 | - | 0,077 |
| 4 to 5 | 223 | 144 | 64,6 | ||
| 2 to 3 | 143 | 107 | 74,8 | ||
| 1 | 11 | 10 | 90,9 | ||
| Family income | |||||
| Without fixed income | 41 | 28 | 68,3 | - | 0,102 |
| Up to 1 minimum wage | 350 | 241 | 68,9 | ||
| 1 to 3 minimum wage | 119 | 78 | 65,5 | ||
| 4 to 6 minimum wage | 11 | 11 | 100,0 | ||
| Water source | |||||
| Natural river, lake or pond | 43 | 31 | 72,1 | - | 0,951 |
| Well | 231 | 159 | 68,8 | ||
| Municipal water | 243 | 165 | 67,9 | ||
| Buy mineral water | 4 | 3 | 75,0 | ||
| Water treatment | |||||
| 1% sodium hypochlorite | 314 | 219 | 69,7 | - | 0,167 |
| Filtered or boiled | 24 | 21 | 87,5 | ||
| Buy mineral water | 4 | 3 | 75,0 | ||
| Not applicable† | 179 | 115 | 64,2 | ||
| Greens and vegetable consumption | |||||
| Yes | 418 | 298 | 71,3 | 1,780 (1,140; 2,778) | 0,013* |
| No | 103 | 60 | 58,3 | ||
| Hygiene of fruits and vegetables | |||||
| Yes | 412 | 294 | 71,4 | 1,246 (0,225; 6,893) | 1,000 |
| No | 6 | 4 | 66,6 | ||
| Meat consumption | |||||
| Yes | 507 | 348 | 68,6 | 0,940 (0,240; 3,683) | 1,000 |
| No | 14 | 10 | 71,4 | ||
| Pork meat consumption | |||||
| Yes | 298 | 209 | 70,1 | 1,166 (0,803; 1,694) | 0,445 |
| No | 223 | 149 | 66,8 | ||
| Consumption of wild animal meat | |||||
| Yes | 296 | 210 | 70,9 | 1,270 (0,875; 1,844) | 0,216 |
| No | 225 | 148 | 65,8 | ||
| Soil contact | |||||
| Yes | 243 | 167 | 68,7 | 1,001 (0,690; 1,451) | 1,000 |
| No | 278 | 191 | 68,7 | ||
| Previous knowledge about toxoplasmosis | |||||
| Yes | 231 | 192 | 83,1 | 1,304 (0,895; 1,899) | 0,183 |
| No | 290 | 166 | 57,2 | ||
| What you heard or where you heard | |||||
| Rat disease or cat disease | 8 | 6 | 75,0 | - | 0,704 |
| Skin disease or serious illness | 2 | 2 | 100,0 | ||
| School or lecture | 6 | 3 | 50,0 | ||
| Tests or health center | 4 | 3 | 75,0 | ||
| Do not remember† | 211 | 149 | 70,6 | ||
| Previous toxoplasmosis test | |||||
| Yes | 56 | 39 | 69,6 | - | 0,606 |
| No | 454 | 313 | 68,9 | ||
| Do not remember | 11 | 6 | 54,5 | ||
| Previous test results for toxoplasmosis | |||||
| Positive | 4 | 4 | 100,0 | - | 0,009* |
| Negative | 17 | 7 | 41,2 | ||
| Do not remember† | 35 | 28 | 80,0 | ||
| Abortion | |||||
| Yes | 83 | 71 | 85,5 | - | 0,011* |
| No | 208 | 131 | 63,0 | ||
| No answer† | 24 | 16 | 66,7 | ||
| Cat as a pet | |||||
| Yes | 170 | 127 | 74,7 | 1,534 (1,018; 2,312) | 0,044* |
| No | 351 | 231 | 65,8 | ||
| Total | 521 | 358 | 68,7 | ||
* Categories not included in statistical analysis; † p < 0.05; - not a 2 x 2 variable.
Associating the results obtained by ELISA and IFAT, the frequency of anti-T. gondii IgG antibodies was 68.7% (358/521): 95.5% (322) of the serum samples were reactive in ELISA and IFAT; 11.4% (21) were reagents only in the ELISA; and 4.4% (15) only on IFAT. Of the 358 IgG samples, 343 (65.8%) were detected by ELISA and 337 (64.7%) by IFAT (Table 2). The comparison of the results obtained in the investigation of anti-T. gondii IgG showed that there was almost perfect agreement between ELISA and IFAT, with Kappa = 0.84, co-positivity of 95.5% and co-negativity of 88.6%. By McNemar's statistical test, the p-value was 0.405, corroborating the agreement between serological techniques.
Table 2 - Frequency of anti-T. gondii antibodies detected by IFAT and ELISA in serum samples from patients treated at the Municipal Laboratory of Oriximiná, Pará sate, Brazil
| ELISA IgG reactive | ELISA IgG non-reactive | Total | |
|---|---|---|---|
| RIFI IgG reactive | 322 (95,5%) | 15 (4,4%) | 337 (64,7%) |
| RIFI IgG non-reactive | 21 (11,4%) | 163 (88,6%) | 184 (35,3%) |
| Total | 343 (65,8%) | 178 (34,1%) | 521 (100,0%) |
| ELISA IgM reactive | ELISA IgM non-reactive | Total | |
| RIFI IgM reactive | 17 (18,5%) | 75 (81,5%) | 92 (17,7%) |
| RIFI IgM non-reactive | - | 429 (100,0%) | 429 (82,3%) |
| Total | 17 (3,7%) | 504 (96,7%) | 521 (100,0%) |
Conventional sign used: - Numeric data equal to zero, not resulting from rounding.
Of the 521 samples analyzed, 92 (17.7%) presented IgG/IgM anti-T.gondii immunoglobulins, which were detected using IFAT. Of these, only 17 were detected using the ELISA (Table 2). The comparison of the results obtained in the investigation of IgM, between the IFAT and ELISA tests showed weak agreement (Kappa = 0.272) and p-value < 0.001 in the McNemar test, confirming the presence of non-random disagreements between these two techniques regarding IgM, with co-positivity of only 18.5% and co-negativity of 100.0%. Four samples that showed only IgM antibodies in IFAT were positive for rheumatoid factor due to the agglutination reaction in the test.
According to the results of Fisher's exact test, shown in table 1, the variables age group, fruit and vegetable consumption, previous results of test for toxoplasmosis, occurrence of abortion and presence of cat as a pet showed a statistically significant association with positivity for T. gondii (p < 0.05). However, the variables resulting from previous test of toxoplasmosis and the occurrence of abortion were removed from the modeling stage, since they imposed enormous restrictions and a consequent decrease in the amount of data in the analysis. The variables number of residents in the household, family income, water treatment, prior knowledge about toxoplasmosis and consumption of wild animal meat were also added in the modeling stage, as their corresponding p-values were lower than the adopted threshold (p ≤ 0.25).
From the data presented in table 3, it is possible to verify that the variables age group, water treatment, presence of cat as a pet and family income were considered those that best explained, together, the risk of infection by T. gondii. Through the OR values, it can be seen that the age group variable was considered a factor that increases the risk of infection (OR > 1), with the risk of infection increasing with age. The water treatment variable appeared as not significant in the model, but its permanence was established because it had a withdrawal p-value less than 0.15, as previously established, with an OR > 1 (although not significant) for cases in which 1% sodium hypochlorite was not used. The presence of a cat as a pet was considered a risk factor (OR > 1) for T. gondii. The variable family income can be seen as a protective factor (OR < 1), probably because the percentage of infected people was higher in the group with the highest family income (100.0%) in relation to the other income groups evaluated. An investigation of possible correlations between income and other variables in the model was carried out, without identifying a clear association with any of them.
Table 3 - Risk factors associated with T. gondii infection in serum samples from patients treated at the Municipal Laboratory of Oriximiná, Pará State, Brazil, verified by multivariate analysis
| Variable | Total samples | Multivariate analysis | Significance (model variable) | |
|---|---|---|---|---|
| OR (IC 95%) | p-value | |||
| Age group | ||||
| Child (≤ 11 years old) | 66 | < 0,001† | < 0,001* | |
| Teenager (12-18 years old) | 48 | 3,730 (1,649; 8,438) | 0,002† | |
| Adult (19-59 years old) | 184 | 12,713 (6,516; 24,804) | < 0,001† | |
| Elderly (≥ 60 years old) | 44 | 36,704 (9,923; 135,758) | < 0,001† | |
| Water treatment | ||||
| 1% sodium hypochlorite | 314 | 0,208 | 0,146* | |
| Filtered or boiled | 24 | 3,552 (0,870; 14,498) | 0,077 | |
| Buy mineral water | 4 | 1,382 (0,082; 23,307) | 0,822 | |
| Cat as a pet | ||||
| No | 234 | 0,033* | ||
| Yes | 108 | 1,951 (1,039; 3,662) | 0,038† | |
| Family income | ||||
| Without fixed income | 27 | < 0,001† | < 0,001* | |
| Up to 1 minimum wage | 224 | 0,238 (0,078; 0,725) | 0,012† | |
| 1 to 3 minimum wage | 82 | 0,349 (0,198; 0,617) | < 0,001† | |
| 4 to 6 minimum wage | 9 | 0,216 (0,098; 0,475) | < 0,001† | |
*Permanence variable ≤ 0,15; † p < 0,05.
DISCUSSION
The positivity for T. gondii among patients seen at the Municipal Laboratory of Oriximiná was verified through the combination of two serological techniques. Regarding the detection of anti-T. gondii IgG antibodies, the ELISA technique showed positivity slightly higher than the IFAT, with a high agreement rate. Greater positivity by ELISA than by IFAT for anti-T. gondii IgG detection was also found in samples from patients in Rio de Janeiro and Colombia19,20. Unlike the investigation of anti-T.gondii IgG immunoglobulins, the detection of IgM antibodies, in this study, occurred mainly by the IFAT technique. Thus, when comparing the techniques, the Kappa index was low, classified as minimal agreement, and the McNemar test also showed low agreement, highlighting the disagreement between the serological techniques in the investigation of this immunoglobulin. Low agreement between IFAT and ELISA serological techniques for the detection of IgM, similar to that evidenced in the serum of patients in Oriximiná, was reported in Rio de Janeiro19.
The lack of complete agreement between IFAT and ELISA was already expected, since the antibodies detected by these serological techniques react with different structures of the protozoan. The IFAT detects antibodies that are produced against surface antigens expressed by the protozoan on its membrane, being more specific; while the indirect ELISA detects antibodies that are produced against cytosolic or metabolic antigens, being more sensitive2. Thus, even though these techniques have a high agreement, it is still not perfect, being necessary to use different associated serological techniques. This fact becomes even more important in the detection of IgM, since the antibodies detected in the IFAT, that is, produced against membrane antigens, tend to be produced early, when compared with the antibodies detected by ELISA2. Although in this study the use of excellent commercial reagents, the lack of perfect agreement between the techniques can also be linked to the reagents and the antigen used in the different stages or even to technical processing. The lack of agreement between IFAT and ELISA evidenced in this study demonstrates the need to use more than one serological technique for the diagnosis of anti-T. gondii antibodies.
It is important to emphasize that the results of serological tests must be carefully analyzed, since the presence of some antibodies can represent a cross reaction, especially with the Fc fractions of the autoantibodies of the rheumatoid factor21. In four samples that presented only IgM tested on IFAT, this factor was present. Rheumatoid factor is a term used to describe a variety of antibodies, with the IgM class being the most frequently detected19. This factor can be produced in patients with chronic arthritis or in cases of infection by other infectious agents. The presence of this factor in serum samples collected from people residing in the Amazon Region, such as those in the city of Oriximiná, should be investigated, given the scarcity of studies in this region.
Despite the sensitivity of immunoenzymatic assays, in the case of IgM screening for toxoplasmosis, the best option, as performed in this study, are the assays based on the immunocapture ELISA, which consist of indirect ELISA assays as described in the notification and investigation protocol: gestational and congenital toxoplasmosis22. This test eliminates or minimizes the possibility of false negatives, due to the issue of high levels of IgG competing with the epitopes for IgM, masking the detection of this antibody; and false positives, due to the question of rheumatoid factors. Even so, in this study, these rheumatoid factors were analyzed taking into account the positive for IgM in the IFAT test, to increase the accuracy of the diagnosis of antibodies by this technique.
From the serological analyses of patients of different age groups treated at the Municipal Laboratory of Oriximiná, the general frequency of anti-T. gondii antibodies was 68.7%. Among these individuals, 51% had a previous infection profile, that is, only IgG was detected, while in 17.7%, IgG/IgM immunoglobulins were detected, a serological profile compatible with the acute infection phase. In people of different age groups from a riverside population of Lábrea, Amazonas State, the general frequency of anti-T. gondii antibodies was 56.7%, consisting of positivity for IgG profile (50.6%) and for IgG/IgM profile (6.1%)6. These frequencies are slightly lower than those observed in the present study.
Higher frequencies than those found in this study were reported in rural areas in Rondônia State (73.3%) and in Pedro Peixoto, Acre State (65.8%), in samples that also included wide age groups23,24. In addition, in population studies in Porto Velho, Rondônia State and Novo Repartimento, Pará State, serological positivities higher than those in this study were also reported, 73.4% and 81.9%, respectively. Lower serological positivity than that of the present study were found in other regions of the country: in patients treated in a clinical analysis laboratory in Goiânia, Goiás State (33.2%)25; in patients treated in a laboratory at Triângulo Mineiro, Minas Gerais State (36%)26; and in individuals residing in Santa Cruz, Rio Grande do Norte State (66.2%)27. Although the cause of T. gondii infection in the studied population is unknown, the high positivity observed in Oriximiná was already expected, since this was the pattern reported in several other studies in different cities and states of the Northern Region mentioned above.
There was a significant difference between the age groups included in the study in the bivariate analysis and logistic regression. It was quite evident that there was a higher frequency of anti-T. gondii antibodies in older age groups than in younger ones. Thus, the greatest chance of detecting anti-T. gondii antibodies was identified in the elderly, followed by adults and then by teenagers, when compared to children. However, gender and number of household members were not considered risk factors. A similar overview of age and gender has been reported in other locations in the Northern Region, such as Monte Negro and Porto Velho, in Rondônia State; Pedro Peixoto, Acre State; and in Novo Repartimento, Pará State9,23,24,28. It is important to note that T. gondii has different forms of transmission; thus, people can become infected by various sources of infection, as they get older, the association of age with infection by this protozoan being generally expected. However, the age variable in T. gondii infection should be observed with caution, as it can act as a confounding factor, masking the identification of other risk factors associated with infection by this protozoan. Unlike Pedro Peixoto, in Acre20, where parasitic infection was not linked to a wealth index, in Oriximiná, through logistic regression, it was possible to verify that patients with higher family income ended up presenting a higher risk of previous infection to the parasite. This fact was already expected, since the higher economic income may favor the greater consumption of meat, which may be contaminated with cysts containing T. gondii bradyzoites.
As demonstrated in this study, the consumption of raw vegetables was associated with the occurrence of infection by T. gondii. A similar situation was evidenced in Monte Negro, Rondônia State19. Information related to the hygiene of food was also analyzed in Oriximiná. The food cleaning was not associated with infection by the protozoan. The variables indirectly associated with the contamination of food, such as water (including type and treatment) and soil, also did not provide information that differed from the other variables, and were not identified as relevant for infection by T. gondii. It is known that, in Oriximiná city, the water treatment is carried out using a disinfectant solution applied by the residents in their water reservoirs. This type of treatment may not be adequate to make T. gondii sporulated oocysts unviable in the water, since this biological form has a double cystic wall that gives high environmental resistance to different chemicals to this structure2. However, even if the chemicals used do not make the oocysts unviable, washing the vegetables with water can facilitate the mechanical removal of the oocysts and, thus, minimize exposure to the parasite.
Still in relation to the diet, the habit of eating meat, including the consumption of pork and wild animals, has been one of the most analyzed variables in epidemiological studies on toxoplasmosis, as it is considered one of the main sources of infection. In Oriximiná, meat consumption, including pork and wild animals, was also investigated, as these are usually part of the diet of people living in the North Region. However, associations regarding different kinds of meat and its way of cooking were not related to infection in the present study. Unlike Oriximiná, in Novo Repartimento was observed that the consumption of meat from wild animals was significantly associated with infection by the protozoan9.
Regarding the previous toxoplasmosis test, it was observed that few people had already done it, and those who did not may have contributed to the occurrence of spontaneous abortion, since many women with anti-T. gondii antibodies reported having aborted before. It is important to note that the Municipal Laboratory of Oriximiná conducts research on anti-T. gondii antibodies upon medical request, which usually occurs in the three gestational trimesters. However, the Laboratory does not always have diagnostic kits, which ends up interrupting the implementation of this service indefinitely.
It was observed that the investigated individuals were unaware of toxoplasmosis. Those who said they knew something about it, presented fragmented, confused or incorrect information. Although the participants were not asked about educational level, it was observed that some of them were illiterate or had low levels of education. This may have favored the lack of knowledge about the disease, which, consequently, would have facilitated the infection of these individuals by protozoa, due to the lack of information on its transmission and prevention. Another factor that should be mentioned is that the low level of education of some study participants may have impaired the understanding of the questions on the form, consequently interfering with the quality of the information obtained, including the questions "previous toxoplasmosis test", as well as "already having heard about toxoplasmosis".
In this study, among the bivariate and multivariate analyses, the one that most called our attention was having a cat as a pet. A similar fact was also reported in studies with pregnant women in Vila Nova and Sevilha de Gurupi and in Araguaína and Colinas, in Tocantins State29,30, and in Novo Repartimento, Pará, in epidemiological studies with different people in the population9. In Pelotas, Rio Grande do Sul State, people who had cats were twice as likely to become infected with T. gondii than those who did not, and this variable, just like in Oriximiná, is associated with infection31. Although cats in Oriximiná had owners, as in the other studies mentioned above, these were semi-domiciled cats, who could access the streets and come into contact with stray cats and other animals, including wild animals. The habit of preying on wild animals may ease the infection of cats by atypical strains of T. gondii. This fact has already been reported mainly in South America, including in the Amazon rainforest in French Guiana32. The free circulation of cats can favor the release of protozoan oocysts in the environment and, consequently, increase the risk of infection of intermediate hosts, such as birds and mammals, including humans. In this context, it is important to note that intermediate hosts can become infected with T. gondii and even reinfect themselves with atypical strains, even if they already have immunity against infection by the protozoan.
From the multivariate analysis of the risk factors observed in the population studied in Oriximiná, it was possible to confirm that there was a significant association between the variables age, family income and having a cat as a pet with seropositivity for T. gondii. This analysis is essential to assess the risk factors associated with infection by this parasite, since several transmission mechanisms are possible. In addition, this parasite is heterogenous and can infect several species of animals, which can be consumed by the human population or by cats. The lack of association between other risk factors and infection by the protozoan was already expected, since patients treated at the Municipal Laboratory of Oriximiná could be exposed to several simultaneous sources of infection that should not be considered in isolation.
Although the following data was not analyzed statistically, a pertinent observation about Oriximiná, which may favor infection by the protozoan, is the city's precarious basic sanitation and the presence of water channels and gutters in its streets. Part of the residential sewage is released through open ditches in various parts of the city. This lack of drainage of residential sewage, through closed pipes, allows animals to access these ditches and cats can eliminate their feces in these places. During this study's fieldwork, it was observed that stray animals, including cats, had free access to these ditches. Thus, T. gondii oocysts can mix with this residue and end up contaminating the environment, which facilitates the transmission of oocysts to susceptible hosts. It is worth mentioning that the climate of this region can also be an important factor in the transmission of the protozoan, since the temperature remains high throughout the year. Basically, there is a rainy season from December to July, which can facilitate the dispersion of oocysts in the environment, exposing people to infection by T. gondii, even those who do not have a cat at home.
Although pregnant women were not the main focus of this study, 25 of them were among the participants. From these, 14 had a previous infection profile verified by serological tests (IgG antibodies), while three had no antibodies against the parasite, eight had a profile compatible with acute infection. These results highlight the need for further studies in Oriximiná including samples from patients who are part of groups at risk for infection, such as pregnant women, newborns, immunocompromised patients, and those with altered visual acuity. It is worth mentioning that congenital transmission of toxoplasmosis has already been confirmed in several states in the Northern Region of Brazil, in addition to cases of symptomatic changes in newborns and cases of ocular toxoplasmosis and infection in patients with the acquired immunodeficiency virus3,30,33,34,35,36,37,38.
At the end of the analysis, the results of the serological tests were sent to the professionals responsible for the Laboratory, to be delivered confidentially to each study participant. Pregnant women were advised to consult the hospital doctor for further instructions about their results.
From these results, it can be observed that, in the city of Oriximiná, there is a need to carry out a program that involves health education activities, aiming to make the population aware of toxoplasmosis; training and updating health professionals, including information on parasites prophylaxis, such as toxoplasmosis; and improvements in the sanitary system, by the implementation of sewage pipes, drinking water treatment, removal of solid waste and environmental drainage. Birth control measures for the cat population is also needed, especially for cats that roam freely in the city.
CONCLUSION
In general, a high frequency of T. gondii seropositive patients treated at the Municipal Laboratory of Oriximiná was evidenced. In addition, it was found that the variables age, presence of cat as a pet, as well as the source of income were associated with infection by this parasite. However, the 100% lack of agreement between the serological techniques used, IFAT and ELISA, evidenced in this study, demonstrates the need to use more than one serological technique for the diagnosis of anti-T. gondii antibodies.
ACKNOWLEDGEMENTS
The study team would like to thank the Municipal Laboratory of Oriximiná, the Rectory of Extension of UFF, the Advanced Unit José Veríssimo of UFF, in Oriximiná, and the Laboratory of Toxoplasmosis and Protozooses of Instituto Oswaldo Cruz/Fiocruz, for the support provided.
REFERENCES
1 Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004 Jun;363(9425):1965-76. [ Links ]
2 Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012 Apr;25(2):64-296. [ Links ]
3 Carmo EL, Almeida EF, Bichara CN, Póvoa MM. Pesquisa de anticorpos anti Toxoplasma gondii em fluidos intra-oculares (humor vítreo e humor aquoso) de pacientes com toxoplasmose ocular, na cidade de Belém, PA. Rev Soc Bras Med Trop. 2005 jan-fev;38(1):77-9. [ Links ]
4 Hill D, Dubey JP. Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect. 2002 Oct;8(10):634-40. [ Links ]
5 Amendoeira MRR, Camillo-Coura LF. Uma breve revisão sobre toxoplasmose na gestação. Sci Med. 2010;20(1):113-9. [ Links ]
6 Vitaliano SN, Mendonça GM, Sandres FAM, Camargo JSAA, Tarso P, Basano SA, et al. Epidemiological aspects of Toxoplasma gondii infection in riverside communities in the Southern Brazilian Amazon. Rev Soc Bras Med Trop. 2015 May-Jun; 48(3):301-6. [ Links ]
7 Dubey JP, Lago EG, Gennari SM, Su C, Jones JL. Toxoplasmosis in humans and animals in Brazil: high prevalence, high burden of disease, and epidemiology. Parasitol. 2012;139:1375-424. [ Links ]
8 Carmo EL, Póvoa MM, Monteiro NS, Marinho RR, Nascimento JM, Freitas SN, et al. Surto de toxoplasmose humana no Distrito de Monte Dourado, Município de Almeirim, Pará, Brasil. Rev Pan-Amaz Saude. 2010 mar;1(1):61-6. [ Links ]
9 Carmo EL, Morais RAPB, Oliveira AS, Figueredo JE, Figueredo MC, Silva AV, et al. Soroepidemiologia da infecção pelo Toxoplasma gondii no Município de Novo Repartimento, Estado do Pará, Brasil. Rev Pan-Amaz Saude. 2016 dez;7(4):79-87. [ Links ]
10 Morais RAPB, Freire ABC, Barbosa DRL, Silva LCT, Pinheiro AF, Costa SS, et al. Surto de toxoplasmose aguda no Município de Ponta de Pedras, Arquipélago do Marajó, Estado do Pará, Brasil: características clínicas, laboratoriais e epidemiológicas. Rev Pan-Amaz Saude. 2016 dez;7(n. esp):143-52. [ Links ]
11 Silva HP. A saúde humana e a Amazônia no século XXI: reflexões sobre os objetivos do milênio. Novos Cad NAEA. 2006 jun;9(1):77-94. [ Links ]
12 Cidade-Brasil.com.br: município de Oriximiná [Internet]. 2019 [citado 2019 ago 4]. Disponível em: Disponível em: https://www.cidade-brasil.com.br/municipio-oriximina.html . [ Links ]
13 Universidade Federal Fluminense. O município de Oriximiná [Internet]. 2015 [citado 2019 mai 3]. Disponível em: Disponível em: http://www.uff.br/?q=node/5357 . [ Links ]
14 Instituto Brasileiro de Geografia e Estatística. Cidades@: Pará, Oriximiná [Internet]. Rio de Janeiro: IBGE; 2019 [citado 2019 ago 4]. Disponível em: Disponível em: https://cidades.ibge.gov.br/brasil/pa/oriximina/panorama . [ Links ]
15 Camargo ME. Introdução às técnicas de imunofluorescência. Rev Bras Patol Clin. 1974; 10(30):143-69. [ Links ]
16 Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159-74. [ Links ]
17 Hosmer DW, Lemeshow S, Sturdivant RX. Applied logistic regression. 3th ed. Hoboken: J. Wiley; 2013. 528 p. [ Links ]
18 Lee KI, Koval JJ. Determination of the best significance level in forward stepwise logistic regression. Commun Stat Simul Comput. 1997;26(2):559-75. [ Links ]
19 Uchôa CMA, Duarte R, Laurentino-Silva V, Alexandre GMC, Ferreira HG, Amendoeira MRR. Padronização de ensaio imunoenzimático para pesquisa de anticorpos das classes IgM e IgG anti-Toxoplasma gondii e comparação com a técnica de imunofluorescência indireta. Rev Soc Bras Med Trop. 1999 nov-dez;32(6):661-9. [ Links ]
20 Cortés LJ, Mancera L. Concordancia entre ELISA e IFI para la determinación de anticuerpos tipo IgG contra Toxoplasma gondii. Infectio. 2009 jun;13(2):76-82. [ Links ]
21 Goeldner I, Skare TL, Reason ITM, Utiyama SRR. Artrite reumatoide: uma visão atual. J Bras Patol Med Lab. 2011 out;47(5):495-503. [ Links ]
22 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância das Doenças Transmissíveis. Protocolo de notificação e investigação: toxoplasmose gestacional e congênita. Brasília: Ministério da Saúde; 2018. 31 p. [ Links ]
23 Cavalcante GT, Aguiar DM, Camargo LMA, Labruna MB, Andrade HF, Meireles LR, et al. Seroprevalence of Toxoplasma gondii antibodies in humans from rural Western Amazon, Brazil. J Parasitol. 2006 Jun;92(3):647-9. [ Links ]
24 Ferreira AM, Vitor RWA, Gazzinelli RT, Melo MN. Genetic analysis of natural recombinant Brazilian Toxoplasma gondii strains by multilocus PCR-RFLP. Infect Genet Evol. 2006 Jan;6(1):22-31. [ Links ]
25 Souza AF, Santos AS, Passos XS, Silva AMTC, Ataides FS. Perfis sorológicos para toxoplasmose de pacientes atendidos em um laboratório de Goiânia, Goiás. RBAC. 2016;48(4):337-40. [ Links ]
26 Maia LP, Gómez-Hernández C, Oliveira KR, Nomeline QSS, Aidar FLM, Ferreira GLS. Soroprevalência de toxoplasmose na região do Pontal do Triângulo Mineiro, Minas Gerais, Brasil. Rev Patol Trop. 2012 out-dez;41(4):457-64. [ Links ]
27 Aloise DA, Coura-Vital W, Carneiro M, Rodrigues MV, Toscano GAS, Silva RB, et al. Seroprevalence and risk factors for human toxoplasmosis in northeastern Brazil. Rev Patol Trop. 2017 Oct-Dec;46(4):307-20. [ Links ]
28 Foschiera AIC, Cartonilho G, Teles CBG. Prevalência da toxoplasmose em pacientes atendidos no laboratório central de saúde pública de Porto Velho-RO. Saber Cient. 2009 jan-jun;2(1): 92-103. [ Links ]
29 Torres FL, Gontijo EEL, Silva MG, Castro AM. Fatores de risco associados a toxoplasmose gestacional nas unidades básicas de saúde dos Setores Vila Nova e Sevilha de Gurupi, Tocantins, Brasil. Rev Cereus. 2014 set-dez;6(3). [ Links ]
30 Rocha EM, Lopes CWG, Ramos RAN, Alves LC. Risk factors for Toxoplasma gondii infection among pregnant women from the State of Tocantins, Northern Brazil. Rev Soc Bras Med Trop. 2015 Nov-Dec;48(6):773-5. [ Links ]
31 Santos LSS, Carvalho AM, Aguiar CLG, Cademartori BG, Farias NAR. Seroprevalence and factors associated with Toxoplasma gondii infection in humans and its relationship with contact with domestic cats (Felis catus) in southern Rio Grande do Sul. Rev Patol Trop. 2015 Apr-Jun;44(2): 135-45. [ Links ]
32 Galal L, Hamidovic A, Dardé ML, Mercier M. Diversity of Toxoplasma gondii strains at the global level and its determinants. Food Waterborne Parasitol. 2019 Jun;15:e00052. [ Links ]
33 Souza SLS, Feitoza PVS, Araújo JR, Andrade RV, Ferreira LCL. Causas de óbito em pacientes com síndrome da imunodeficiência adquirida, necropsiados na Fundação de Medicina Tropical do Amazonas. Rev Soc Bras Med Trop. 2008 mai-jun;41(3):247-51. [ Links ]
34 Bichara CNC, Canto GAC, Tostes CL, Freitas JJS, Carmo EL, Póvoa MM, et al. Incidência de toxoplasmose congênita na cidade de Belém, estado do Pará, norte do Brasil, através de um programa de triagem neonatal: resultados preliminares. Rev Soc Bras Med Trop. 2012 jan-fev;45(1):122-4. [ Links ]
35 Lopes FMR, Gonçalves DD, Mitsuka-Breganó R, Freire RL, Navarro IT. Toxoplasma gondii infection in pregnancy. Braz J Infect Dis. 2007 Oct;11(5):496-506. [ Links ]
36 Pereira DAP, Maia BP, Seto IIC, Bichara CNC. Infecção congênita em pacientes matriculados em programa de referência materno infantil. Rev Para Med. 2015 jan-mar;29(1):31-8. [ Links ]
37 Silva MG, Vinaud MC, Castro AM. Prevalence of toxoplasmosis in pregnant women and vertical transmission of Toxoplasma gondii in patients from basic units of health from Gurupi, Tocantins, Brazil, from 2012 to 2014. PLoS One. 2015 Nov;10(11):e0141700. [ Links ]
38 Miranda KCI, Corrêa VC, Martins ND, Corrêa FVS, Furlaneto IP. Prevalência da toxoplasmose em gestantes no Oiapoque-Amapá, Fronteira com a Guiana Francesa. Braz J Hea Rev. 2019 jul-ago;2(4):2825-34. [ Links ]
How to cite this article / Como citar este artigo: Ramos RCF, Palmer JPS, Dib LV, Lobão LF, Pinheiro JL, Santos CR, et al. Seropositivity and risk factors associated with Toxoplasma gondii infection in patients treated at the Municipal Laboratory of Oriximiná, Pará State, Brazil. Rev Pan Amaz Saude. 2021;12:e202100476. Doi: http://dx.doi.org/10.5123/S2176-6223202100476
Received: September 11, 2019; Accepted: October 20, 2020










 text in
text in 
 Curriculum ScienTI
Curriculum ScienTI