Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Pan-Amazônica de Saúde
Print version ISSN 2176-6215On-line version ISSN 2176-6223
Rev Pan-Amaz Saude vol.12 Ananindeua 2021 Epub June 02, 2021
http://dx.doi.org/10.5123/s2176-6223202100620
ORIGINAL ARTICLE
Trematodes emerging from freshwater mollusks collected in ditches, in the Municipality of Peruíbe, São Paulo State, Brazil
1 Superintendência de Controle de Endemias, Programa de Aprimoramento Profissional, Centro Regional de São Vicente, Laboratório de Malacologia, São Vicente, São Paulo, Brasil
2 Superintendência de Controle de Endemias, Centro Regional de São Vicente, Laboratório de Malacologia, São Vicente, São Paulo, Brasil
OBJECTIVES:
Identify freshwater mollusks and trematode larvae from drainage ditches in the Municipality of Peruíbe, São Paulo State, Brazil, and describe the morphology of emerged larvae.
MATERIALS AND METHODS:
Mollusks were collected from 53 ditches. In the laboratory, the specimens were morphologically identified, and parasitological analysis was carried out to determine and describe the larvae.
RESULTS:
5,969 mollusks belonging to the families Planorbidae, Lymnaeidae, Physidae, Thiaridae, and Ampullariidae were collected. The parasitological analysis of the mollusks revealed nine different larvae corresponding to the following seven types of cercariae: xiphidio cercariae, echinostome cercariae, strigea cercariae, brevifurcate pharyngeate distome cercariae, brevifurcate apharyngeate distome cercariae, pleurolophocercous cercariae, and amphistome cercariae. Biomphalaria tenagophila (d'Orbigny, 1835) was susceptible to eight of the nine cercariae found, and six specimens of mollusks were parasitized by Schistosoma mansoni Sambon, 1907.
CONCLUSION:
It is fundamental to establish malacological control and surveillance programs in vulnerable areas when mollusks, intermediate host of parasites of medical and veterinary importance, colonize these environments, especially when infected with S. mansoni larvae.
Keywords: Cercariae; Mollusks; Schistosomiasis; Trematodes
INTRODUCTION
Freshwater gastropod mollusks have representatives in several families of medical and veterinary importance. They have been found in different types of water collections1. The main families of these mollusks are Planorbidae, Lymnaeidae, Physidae, Thiaridae, Ampullariidae, Ancylidae, and Hydrobiidae, and they play the role of intermediate hosts for digenetic trematodes1,2.
Among the planorbids, the genus Biomphalaria Preston, 1910 stands out in Brazil with 10 described species and one subspecies. Eight species were identified in São Paulo State. Three of them were natural hosts of the trematode Schistosoma mansoni Sambon, 1907, Biomphalaria glabrata (Say, 1818), Biomphalaria straminea (Dunker, 1848), and Biomphalaria tenagophila (d'Orbigny, 1835); one was a potential host species of S. mansoni, Biomphalaria peregrina (d'Orbigny, 1835); and four were non-host species of S. mansoni, Biomphalaria occidentalis (Paraense, 1981), Biomphalaria intermedia (Paraense & Deslandes, 1962), Biomphalaria oligoza (Paraense, 1975), and Biomphalaria schrammi (Crosse, 1984)1,2,3.
In São Paulo State, schistosomiasis has a low prevalence epidemiological profile, but with large areas of indigenous transmission and imported cases, covering the regions of the Vale do Ribeira de Iguape, Baixada Santista, Metropolitan Region of Campinas, São Paulo Metropolitan Region, Vale do Paraíba, and Litoral Norte4,5.
Diseases caused by trematodes are transmitted by skin penetration of larvae and ingestion of contaminated food6. In the first case, for example, by the larvae of S. mansoni and, in the second one, by the ingestion of water and food, such as raw or undercooked fish and crustaceans and unwashed vegetables, which may result in several diseases as echinostomiasis paragonimiasis, clonorchiasis, cercariae dermatitis in humans, and fascioliasis7,8,9,10.
The studies on parasite-host interactions are based on fauna surveys considering the trematode-mollusk system. This research model is widely used in studies in Brazil and other countries11,12,13.
Despite the knowledge acquired, it is necessary to describe several larvae, associate them with adult forms, and know aspects related to the biological cycle and others. Only then will it be possible to clarify intermediate hosts' roles in transmitting diseases14,15.
The present study aims to identify freshwater mollusks and trematode larvae from drainage ditches in the municipality of Peruíbe, São Paulo State, and to describe the morphology of the larvae eliminated by those mollusks.
MATERIALS AND METHODS
STUDY AREAS
The mollusks were collected from October to December 2016 and from April to July 2017, in peridomicile irrigation ditches of three areas of Peruíbe, on the southern coast of São Paulo (Figure 1). The selected areas were: A - Nova Peruíbe: 24°17'50"S, 47°1'24"W (peripheral area) with 14 ditches; B - Jardim Caraguava: 24°17'42"S, 47°0'56"W (peripheral area) with 28 ditches; and C - Ruínas: 24°16'8"S, 46°56'12"W (urban area) with 11 ditches. All areas were georeferenced using the GPS (global position system) eTrex Summit® (Garmin).
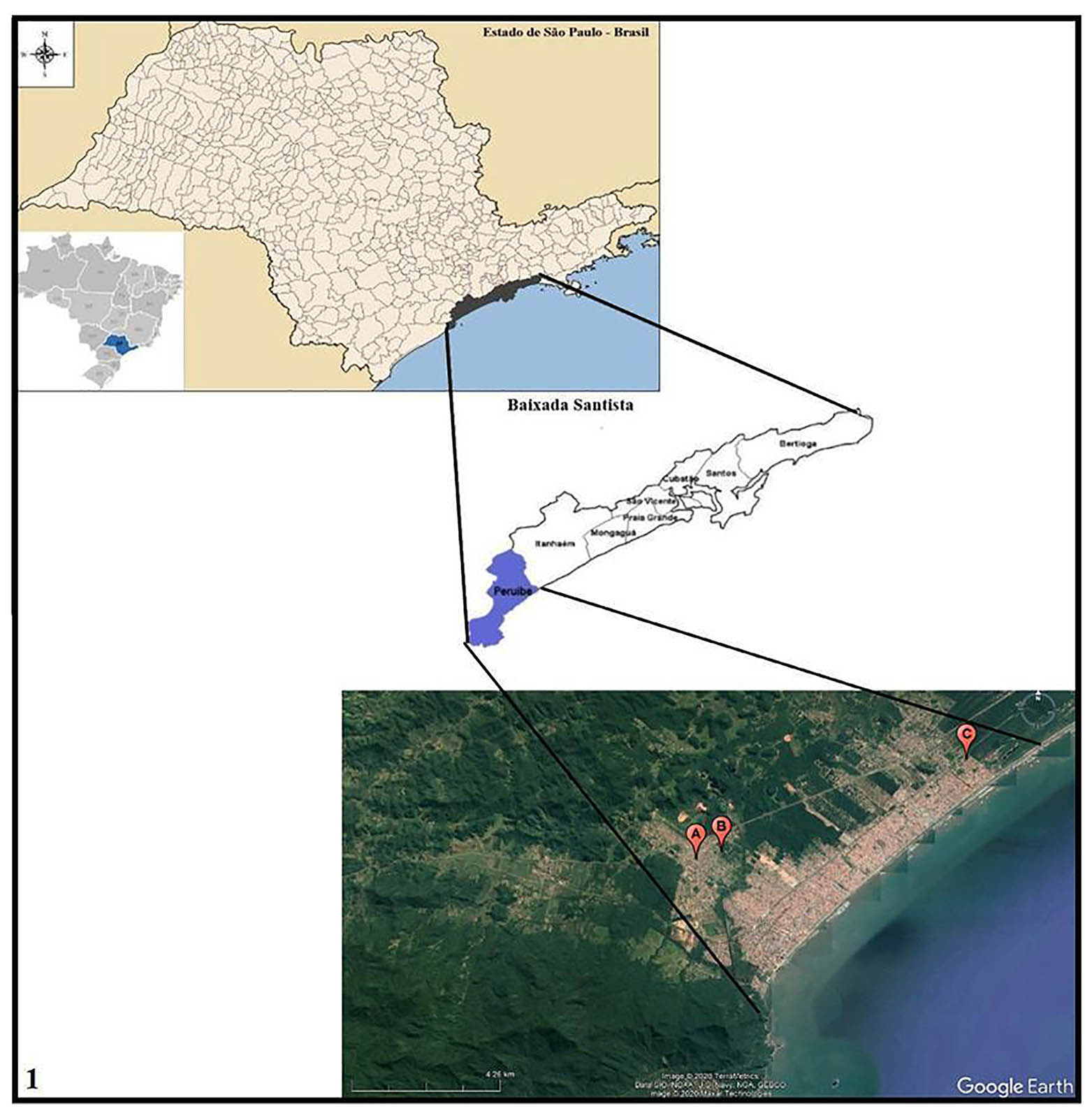
Source: Tabwin version 4.1.5 - Datasus; Google Earth. Image produced by Nayla Zanella Carramão.
Area A: Nova Peruíbe; Area B: Jardim Caraguava; Area C: Ruínas.
Figure 1 - Location of Peruíbe in Baixada Santista, São Paulo State, Brazil, and satellite image of malacological collection areas
Area C (Ruínas) was selected based on the environmental similarities with areas A (Nova Peruíbe) and B (Jardim Caraguava), such as the presence of irrigation ditches with water and vegetation in the peridomicile, as well as insect larvae, tadpoles, mollusks, and some fishes. Around the ditches, some animals were noticed, such as dogs, cats, birds (black vultures, ducks, chickens), and horses. All areas had basic sanitation; however, sanitary effluents were discharged into the ditches, contaminating the soil due to the lack of paving.
COLLECTING MOLLUSKS
It was not necessary to request a license from the Brazilian Institute for the Environment and Renewable Natural Resources to carry out the collections, as the Endemics Control Superintendence (Superintendência de Controle de Endemias - SUCEN) is responsible for the surveillance and control actions of vectors and intermediate hosts1.
The methodology used in the collections followed the SUCEN's guide: Procedimento Operacional Padrão para o Programa de Vigilância e Controle da Esquistossomose Mansônica16. Mollusks were collected along the ditch, three samples at each 100 m stretch.
A resistant plastic cup (200 mL) was considered a sample, with approximately 20 to 30 mollusks, depending on the animal's size. Some aquatic vegetation was used to maintain the humidity inside the cup and, it was sealed with gauze. The samples were identified as to the date of collection, sample number, locality name (neighborhood), ditch name (same as the name of the street), and the ditch stretch number.
The collections were performed with the aid of a collecting shovel, consisting of a wire mesh screen (2 mm) at right angles, with a long wooden handle and a 30 cm stainless steel straight-tipped forceps. All samples were packed in plastic boxes to be transported safely at the end of the collections.
TREATMENT OF MOLLUSKS
The initial procedures took place in the afternoon, as soon as the field samples arrived at the laboratory. Mollusks were quantified and separated by genus, according to the conchological criteria1. After screening, the animals were grouped according to their genus. Each container received up to 15 mollusks, chlorine-free water, and a small amount of fresh lettuce.
PARASITOLOGICAL EXAMINATION
The examinations for assessing cercariae infection in mollusks occurred individually in wells of the culture plate with water, except animals of larger diameter, that were placed in small transparent glass containers (7 cm). The mollusks were exposed to artificial light (photostimulation) for 2 h, always during the day.
The emerged larvae forms from the mollusks were observed in the same cell culture plates they were, which were placed on the base of the stereoscopic microscope in 10-15x magnification. For a better detail of the larval forms, it was necessary to place the larvae between a microscope slide and cover glass to be analyzed under the optical microscope.
Parasitological research was completed with the crushing of mollusks, aiming to find possible cercariae in their internal organs (viscera). For this purpose, an 8.0/12.0 cm glass plate was used, which received up to five mollusks, depending on the animal's size; and, between two plates, the shells were crushed with light pressure16,17. The global and specific infection rates of the mollusks were determined after exams, according to the method described by Ruiz18.
PREPARATION OF THE MICROSCOPE SLIDES
The larvae found in the water and viscera of the crushed mollusks were captured with a pipette and transferred to microscope slides with a drop of water to be examined fresh and, subsequently, analyzed under an optical microscope with the lugol (0.1%) and neutral red (0.05%) stains. The larvae were photographed in 40x magnification, except for Apharyngostrigea sp., a sturdier larva, which was photographed in 10x magnification.
The morphological identification of the larvae was based on the following criteria: body and tail shape; size and position of their suckers; the presence or absence of specialized structures, such as oral stylets and ocelli; the presence of pharynx; among other structures, as shown by the taxonomic keys of Pinto and Melo9, Martorelli et al.15, Naruto19, Frandsen and Christensen20, Shell21, and Boaventura et al.22.
IDENTIFICATION OF BIOMPHALARIA SPP.
After the initial parasitological examinations, two or three specimens of Biomphalaria spp. that did not release cercaria were separated for species identification, according to the protocols of the Brazilian Ministry of Health1, Deslandes23, Pan American Health Organization24, and Paraense25.
The larvae identification was made, and the pictures were taken at the Malacology Laboratory of the Regional Center of São Vicente/SUCEN.
RESULTS
MOLLUSKS COLLECTED AND IDENTIFIED
A total of 5,969 mollusks from 53 ditches distributed in the three areas were collected and examined: 1,163 mollusks in area A; 3,841 in area B; and 965 in area C. The identification of mollusks revealed B. tenagophila, B. straminea, Drepanotrema sp. (Planorbidae), Lymnaea sp. (Lymnaeidae), Physa sp. (Physidae), Pomacea sp. (Ampullariidae), and Melanoides tuberculata (Müller, 1774) (Thiaridae) (Table 1).
Table 1 - Frequency of freshwater mollusks collected in three areas of Peruíbe, São Paulo State, Brazil, 2016-2017
| Mollusks | Area A | Area B | Area C |
|---|---|---|---|
| N | N | N | |
| Biomphalaria tenagophila | 962 | 3.592 | 631 |
| Biomphalaria straminea | - | - | 4 |
| Melanoides tuberculata | 19 | 4 | 163 |
| Physa sp. | 169 | 232 | 124 |
| Lymnaea sp. | - | 6 | 39 |
| Drepanotrema sp. | - | 3 | - |
| Pomacea sp. | 13 | 4 | 4 |
| Total | 1,163 | 3,841 | 965 |
Area A: Nova Peruíbe; Area B: Jardim Caraguava; Area C: Ruínas. Conventional signal used: - Numerical data equal to zero, not resulting from rounding.
IDENTIFICATION OF TREMATODES AND INFECTION RATES
Nine larval forms were recorded and classified into seven morphological types, i.e.: xiphidio cercaria - Cercaria lutzi (Ruiz, 1943); echinostome cercaria - Cercaria granulifera (Lutz, 1924) and Echinostoma sp.; strigea cercaria - Cercaria caratinguensis (Ruiz, 1953) and Apharyngostrigea sp.; brevifurcate pharyngeate distome cercaria - Cercaria ocellifera (Lutz, 1917); brevifurcate apharyngeate distome cercaria - Cercaria blanchardi (Pirajá da Silva, 1912) or larva of S. mansoni; pleurolophocercous cercaria - Centrocestus formosanus (Nishigori, 1924); and amphistome cercaria - Amphistoma sp.. Larvae C. lutzi, C. granulifera, C. caratinguensis, and C. ocellifera appeared more frequently in this study (Table 2).
Table 2 - Frequency of infected mollusks, according to larval forms and infection rate per area surveyed, in Peruíbe, São Paulo State, Brazil, 2016-2017
| Larval forms | Infected mollusks | Area A | Area B | Area C | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Cercaria lutzi | Biomphalaria tenagophila | 9 | 0.93 | 324 | 9.02 | 16 | 2.53 |
| Physa sp. | 1 | 0.59 | - | - | - | - | |
| Cercaria granulifera | Biomphalaria tenagophila | 4 | 0.41 | 59 | 1.64 | 25 | 3.96 |
| Echinostoma sp. | Biomphalaria tenagophila | - | - | 1 | 0.02 | - | - |
| Cercaria caratinguensis | Biomphalaria tenagophila | 4 | 0.41 | 38 | 1.05 | - | - |
| Apharyngostrigea sp. | Biomphalaria tenagophila | - | - | - | - | 3 | 0.47 |
| Cercaria ocellifera | Biomphalaria tenagophila | - | - | 9 | 0.25 | 2 | 0.31 |
| Cercaria blanchardi | Biomphalaria tenagophila | - | - | 6 | 0.16 | - | - |
| Centrocestus formosanus | Melanoides tuberculata | 1 | 5.26 | - | - | - | - |
| Anfistoma sp. | Biomphalaria tenagophila | - | - | 1 | 0.02 | - | - |
Area A: Nova Peruíbe; Area B: Jardim Caraguava; Area C: Ruínas. Conventional signal used: - Numerical data equal to zero, not resulting from rounding.
Among the specimens of B. tenagophila collected in area B (Jardim Caraguava), six specimens carried S. mansoni larvae, representing an infection rate of 0.16%. However, the highest rates of B. tenagophila infection were with C. lutzi (9.02%) in area B, and C. granulifera (3.96%) in area C (Ruínas). When compared by the area surveyed, mollusk infection rates resulted in 11.40%, 4.76%, and 1.63% for Jardim Caraguava, Ruínas, and Nova Peruíbe, respectively (Table 2).
MORPHOLOGICAL DESCRIPTION OF LARVAE FOUND IN PARASITOLOGICAL EXAMS
Two different larvae of echinostome cercaria (Figure 2: A and D) were observed. One of them, called Echinostoma sp., presented: natatory membrane in the single tail (Figure 2: B); oral and ventral suckers of similar sizes; several cystogenic cells scattered throughout the body; the presence of the esophagus and two long intestinal caeca, which reach the posterior region of the body (Figure 2: A). Larvae cultivated in brown redia (Figure 2: C).
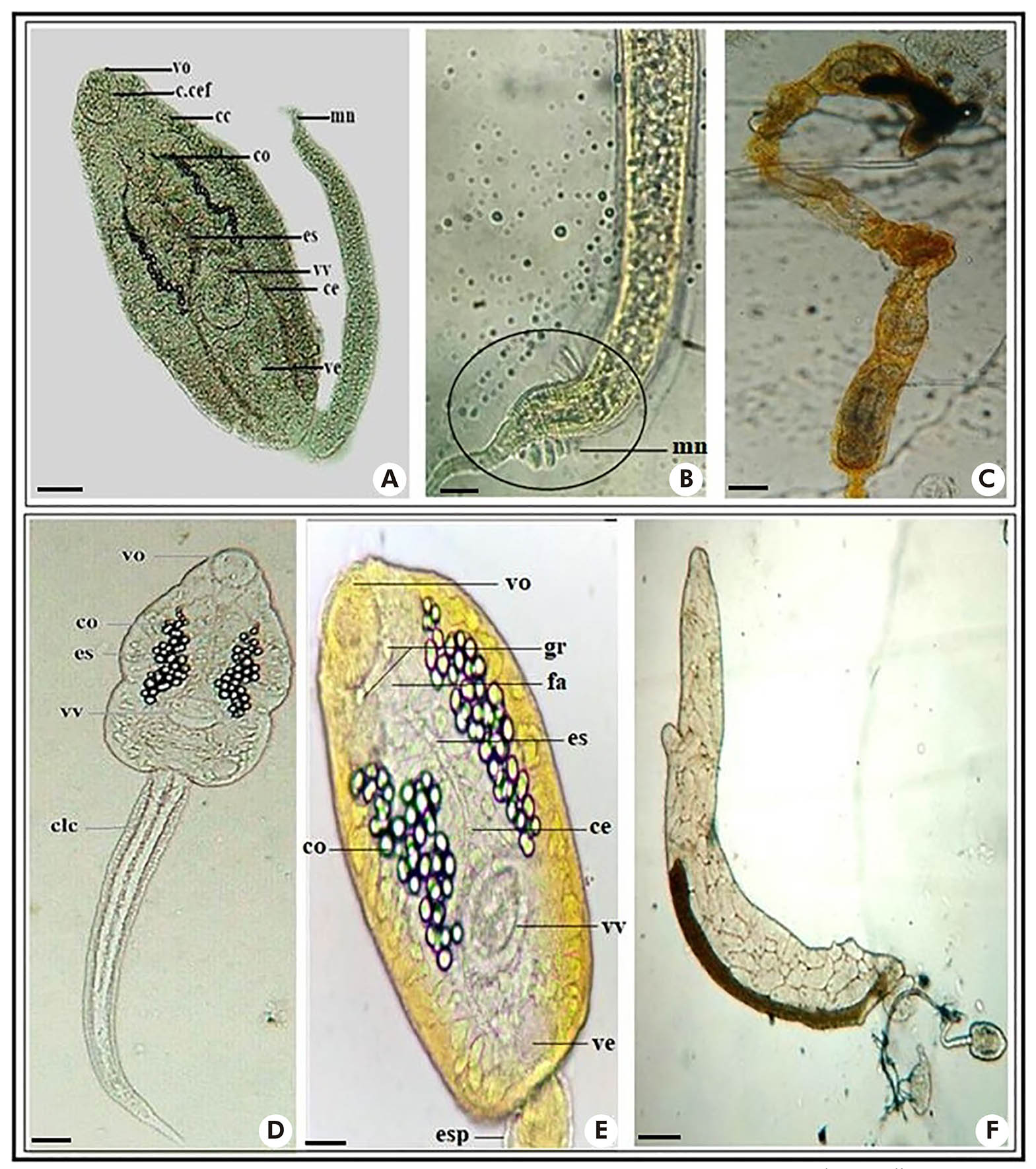
Photos: Nayla Zanella Carramão.
A: Body and tail of Echinostoma sp.; B: Natatory membrane of Echinostoma sp.; C: Brownish redia of Echinostoma sp.; D: Body and tail of Cercaria granulifera; E: Structures of the body and tail of the Cercaria granulifera; F: Brownish redia of Cercaria granulifera. Dye used - Diluted Lugol 0.1% (A, B, E); Without dye - fresh examination (D, F). Scale: 50 μm. vo: Oral sucker; fa: Pharynx; gr: Granules; c.cef: Cephalic collar; cc: Cystogenic cells; co: Concretions; es: Esophagus; vv: Ventral sucker; ce: Caeca; ve: Excretory bladder; mn: Natatory membrane; clc: Caudal cells; esp: Spines.
Figure 2 - Larvae of echinostome cercaria
The second echinostome cercaria (Figure 2: D and E) presented: muscular pharynx, esophagus long, and bifurcated in two intestinal caeca, reaching the posterior region of the body; excretory bladder on each side of the body, with collecting ducts that have, in the most dilated parts, 35-45 spherical refringent granulations or easily visible concretions; oral and ventral suckers; the presence of two refringent granules in front of the pharynx and near the base of the oral sucker (Figure 2: E); simple, long, and narrow tail covered by spines. These characteristics are from C. granulifera, the larval form of Paryphostomum segregatum (Dietz, 1909). Development in redias8 (Figure 2: F).
A larva of xiphidio cercaria, C. lutzi (Figure 3: A and B), presented: oral sucker well-developed and stylet, and ventral sucker in the median region of the body; easily observed penetration glands, five on each side of the body; excretory bladder Y-shaped and simple tail. Development in sporocysts (Figure 3: C).

Photos: Nayla Zanella Carramão.
A, B: Body and tail of Cercaria lutzi; C: Sporocysts of Cercaria lutzi; D: Body and tail of Cercaria caratinguensis; E: Body and tail of Apharyngostrigea sp. Fresh examination. Scale: 50 μm. ve: Excretory bladder (Y); eo: Oral stylet; vo: Oral sucker; gp: Penetration glands; vv: Ventral sucker; oc: Ocelli; esp: Spines; clc: Caudal cells; f: Furca; pg: Genital primordium.
Figure 3 - Larvae of xiphidio cercaria (A, B, C) and strigeo cercaria (D, E)
Two strigea cercaria larvae (Figure 3: D and E) were found, one of them similar to C. caratinguensis, with: large body; oral and ventral suckers, the latter was located in the middle part of the body; ocelli without pigmentation; long bifurcated tail, with furcations flattened laterally and longer than the stem, and five pairs of cells were observed in its interior. Development in sporocysts20,21.
The second strigea cercaria (Figure 3: E) presented features similar to the larva Apharyngostrigea: large and oval body; oral and ventral suckers (spherical); pharynx; two bag-shaped penetration glands on each side of the body, just below the oral sucker; genital primordium near the tail; bifurcated tail, with medium and wide furcations, flattened dorsum ventrally, and a markedly globular caudal trunk; and brown-colored granules inside the body and tail. Development in sporocysts20,21.
The larvae of the brevifurcate pharyngeate distome type (Figure 4: A and B) resemble C. ocellifera, of the family Clinostomidae, with: dorsal membrane in the elongated body; pigmented ocelli; large and oval oral sucker, ventral sucker not visualized; long tail and bifurcated in short furcations. Development in rediae8.

Photos: Nayla Zanella Carramão.
A: Body and tail of Cercaria ocellifera; B: Body of Cercaria ocellifera; C: Body and tail of Centrocestus formosanus; D: Body and tail of Cercaria blanchardi (Schistosoma mansoni); E: Body and tail of Anfistoma sp.. Dyes: neutral red 0.05% (C) and lugol 0.1% (D, E). Scale: 50 μm. vo: Oral sucker; oc: Ocelli; md: Dorsal membrane; f: Furca; vv: Ventral sucker; fa: Pharynx; ce: Caeca; ve: Excretory bladder; gp: Penetration glands.
Figure 4 - Larval types of brevifurcate pharyngeate distome cercaria, pleurolophocercous cercaria and amphistome cercaria - with pigmented ocelli (A, B, C, E); and brevifurcate apharyngeate distome - without pigmented ocelli (D)
The amphistome cercaria type larva (Figure 4: E) presented: elongated body, with scattered cystogenic cells and two pigmented ocelli; large oral and ventral suckers, with the ventral sucker located in the posterior region of the body; and simple tail. Development in redias8,26.
The pleurolophocercous cercaria larva (Figure 4: C) presented characteristics of C. formosanus, with: oval body and numerous cystogenic cells; large oral sucker, and ventral sucker difficult to visualize; muscular pharynx; two long intestinal cecae, below the ocelli and excretory bladder in the back part of the tail; simple tail with small natatory membrane. Development in rediae27,28.
The larva C. blanchardi, a brevifurcate apharyngeate distome cercaria type (Figure 4: D), presented: elongated body; developed oral sucker and small ventral sucker, located in the subequatorial area of the body; adhesion and penetration glands; absent pharynx; cylindrical tail, bifurcated in short furcations. Development in sporocysts19.
DISCUSSION
The results allowed us to identify the larval forms and intermediate host mollusks of the trematodes, expanding the knowledge about the epidemiological and sanitary conditions of the areas surveyed in Peruíbe. It is emphasized that, although there is basic sanitation, the ditches were receiving sanitary effluents, favoring the transmission of diseases and possibly schistosomiasis.
The malacological research resulted in 5,969 mollusks collected. The species B. tenagophila predominated in all areas, and Physa sp. was the second numerically superior. It is important to consider that the malacological fauna is closely related to environmental factors, and some taxonomic groups may be more sensitive to certain environmental disturbances than others9. Because of these biological characteristics, mollusks are widely used to evaluate and monitor environmental conditions12,13.
When compared globally, the infection rate was higher in the Jardim Caraguava (area B) with 11.4%. Regarding the specific infection rate, B. tenagophila was the most parasitized species, susceptible to eight of the nine larval forms found (Table 2). Echinostoma sp., Apharyngostrigea sp., C. formosanus, and Anfistoma sp. were eliminated by few mollusks. These data may indicate the influence of environmental factors on trematode larvae; however, such aspects may be elucidated with new studies.
Morphological studies revealed six species of trematode larvae, and three larvae did not receive specific identification, such as Echinostoma sp., Apharyngostrigea sp., and Anfistoma sp. (Table 2). According to Locke et al.29, the species cannot be identified just by morphology. It is necessary to combine molecular tools from the larval stages and associate them with the known adult forms.
Regarding C. lutzi (Figure 3: A and B), this larva was associated with the parasite Pneumonoeces neivai (Travassos & Artigas, 1927) found in anuran lungs30,31. Trematodes of the family Echinostomatidae are intestinal parasites of birds, reptiles, and mammals, including humans with case reports in Asia9,14. The echinostome cercaria C. granulifera (Figure 2: D and E) was considered the larval form of P. segregatum. Ruiz32 verified the cystic forms in the pharynx of tadpoles, fish, and mollusks and the adult form of the parasite in a black vulture.
The larva Anfistoma sp. (Figure 4: E), from the Paramphistomoidea group, has veterinary importance, especially for ruminants33. The larva Anfistoma lunatum was associated with the parasite Zygocotyle lunata (Diesing, 1836), found in ruminant mammals and birds, such as free-range chicken (Gallus gallus domesticus). According to Barbosa26, the encystation of the larva occurs in substrates of the external environment, such as molluscum shell, crustacean, tadpole integument, vegetation, or any object.
C. formosanus (Figure 4: C) has been associated with centrocestiasis in humans in Asia (Taiwan and the Philippines), acquired through consumption of raw fish with metacercariae26,33,34. Larvae identified as C. caratinguensis and Apharyngostrigea sp. (Figure 3: D and E) belong to the Strigeidae family. The parasites were found in birds and mammals; and metacercariae in fish, mollusks, and amphibians35.
C. ocellifera (Figure 4: A and B) is considered the larval form of Clinostomum heluans Braun, 1989, of the Clinostomatidae family, a parasite of reptiles and aquatic birds, also found in tadpoles, fish, and mollusks, its intermediate hosts31.
Finally, S. mansoni (C. blanchardi) larvae were found in six specimens of B. tenagophila from ditches in Jardim Caraguava, an area affected by floods. Residents report that the floods carry mollusks into the houses, making physical contact inevitable, demonstrating that this is yet another situation of environmental vulnerability that can cause the transmission of several diseases, including schistosomiasis. According to Artigas et al.36, cases of schistosomiasis have been reported in Peruíbe since 1966.
CONCLUSION
The presence of trematode larvae in the studied area indicates the existence of active foci that require attention, especially due to the presence of mollusks carrying S. mansoni larvae. Thus, malacological control and surveillance measures are essential in vulnerable areas such as Peruíbe, whose mollusk fauna is composed of families of medical and veterinary importance.
ACKNOWLEDGMENTS
To the field team for the excellent work; and to the technicians of the Malacology Laboratory, who collaborated with this research, especially to Antonio da Silva
REFERENCES
1 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Vigilância e controle de moluscos de importância epidemiológica: diretrizes técnicas: Programa de Vigilância e Controle da Esquistossomose (PCE). 2. ed. Brasília: Ministério da Saúde; 2008. (Série A. Normas e manuais técnicos) [Link] [ Links ]
2 Ohlweiler FP, Takahashi FY, Guimarães MCA, Gomes SR, Kawano T. Manual de gastrópodes límnicos e terrestres do Estado de São Paulo associados às helmintoses. Porto Alegre: Redes; 2010. [ Links ]
3 Palasio RGS, Guimarães MCA, Ohlweiler FP, Tuan R. Molecular and morphological identification of Biomphalaria species from the state of São Paulo, Brazil. ZooKeys. 2017 Apr;668:11-32. Doi: 10.3897/zookeys.668.10562 [Link] [ Links ]
4 Secretaria de Estado da Saúde (SP). Centro de Vigilância Epidemiológica "Prof. Alexandre Vranjac. Esquistossomose [Internet]. São Paulo; 2021 [citado 2021 jan 12]. Disponível em: Disponível em: https://www.saude.sp.gov.br/resources/cve-centro-de-vigilancia-epidemiologica/areas-de-vigilancia/doencas-de-transmissao-por-vetores-e-zoonoses/dados/esquisto/esquisto_dados.pdf . [ Links ]
5 Secretaria de Estado da Saúde (SP). Centro de Vigilância Epidemiológica "Prof. Alexandre Vranjac. Esquistossomose mansoni no Estado de São Paulo: novas estratégias de controle e critérios para eliminação da transmissão [Internet]. São Paulo; 2020 [citado 2020 abr 7]. Disponível em: Disponível em: http://www.saude.sp.gov.br/resources/cve-centro-de-vigilancia-epidemiologica/areas-de-vigilancia/doencas-transmitidas-por-agua-e-alimentos/eventos/2esqui11_estrategias.pdf . [ Links ]
6 Rey L. Bases da parasitologia médica. 3. ed. Rio de Janeiro: Guanabara Koogan; 2010. [ Links ]
7 Farahnak A, Vafaie-Darian R, Mobedi I. A faunistic survey of cercariae from fresh water snails: Melanopsis spp. and their role in disease transmission. Iranian J Publ Health. 2006;35(4):70-4. [ Links ]
8 Pinto HA. Biologia e taxonomia de trematódeos transmitidos por moluscos dulciaquícolas na represa da Pampulha, Belo Horizonte, Minas Gerais, Brasil [tese]. Belo Horizonte (MG): Universidade Federal de Minas Gerais, Instituto de Ciências Biológicas; 2013. 299 p. [ Links ]
9 Pinto HA, Melo AL. Larvas de trematódeos em moluscos no Brasil: panorama e perspectivas após um século de estudos. Rev Patol Trop. 2013 out/dez;42(4):369-86. Doi: 10.5216/rpt.v42i4.27922 [Link] [ Links ]
10 Neves DP, coordenador. Parasitologia humana. 13. ed. São Paulo: Atheneu; 2016. [ Links ]
11 Madsen H, Hung NM. Reprint of "An overview of freshwater snails in Asia with main focus on Vietnam". Acta Trop. 2015 Jan;141(Pt B):372-84. Doi: 10.1016/j.actatropica.2014.10.014 [Link] [ Links ]
12 Soldánová M, Selbach C, Sures B, Kostadinova A, Pérez-del-Olmo A. Larval trematode communities in Radix auricularia and Lymnaea stagnalis in a reservoir system of the Ruhr River. Parasit Vectors. 2010 Jun;3:56. Doi: 10.1186/1756-3305-3-56 [Link] [ Links ]
13 Dodangeh S, Daryani A, Sharif M, Gholami S, Kialashaki E, Moosazadeh M, et al. Freshwater snails as the intermediate host of trematodes in Iran: a systematic review. Epidemiol Health. 2019;41:e2019001. Doi: 10.4178/epih.e2019001 [Link] [ Links ]
14 Souza MAA, Melo AL. Caracterização de larvas de trematódeos emergentes de moluscos gastrópodes coletados em Mariana, Minas Gerais, Brasil. Iheringia, Ser Zool. 2012 mar;102(1):11-8. Doi: 10.1590/S0073-47212012000100002 [Link] [ Links ]
15 Martorelli SR, Alda P, Marcotegui P, La Sala LF, Montes MM. Larval digeneans in Biomphalaria snails from the Salto Grande Dam area in the Uruguay River. Publicacion del Laboratorio de Helmintos y Parasitos de Crustaceos del Cepave; 2013. 13 p. [Link] [ Links ]
16 Secretaria de Estado da Saúde (SP). Superintendência de Controle de Endemias. Manual de procedimento operacional padrão para o Programa de Vigilância e Controle da Esquistossomose Mansônica: POPESQUISTO. São Paulo; 2018. [Link] [ Links ]
17 Carvalho OS, Passos LKJ, Mendonça CLF, Cardoso PCM, Caldeira RL. Moluscos brasileiros de importância médica. 2. ed. Belo Horizonte: FIOCRUZ, Centro de Pesquisas René Rachou; 2014. (Série Esquistossomose; 16). [Link] [ Links ]
18 Ruiz JM. Índices cercáricos específicos do Schistosoma mansoni verificados em Neves e Mariana, Estado de Minas Gerais. Mem Inst Butantan. 1952;24(1):63-5. [ Links ]
19 Naruto T. Guia para identificação de cercárias. São Paulo: Superintendência de Controle de Endemias do Estado de São Paulo; 1984. 61 p. [ Links ]
20 Frandsen F, Christensen NO. An introductory guide to the identification of cercariae from African freshwater snails with special reference to cercariae of trematode species of medical and veterinary importance. Acta Trop. 1984 Jun;41(2):181-202. [ Links ]
21 Schell SC. How to know the trematodes. Dubuque (USA): W. C. Brown Company; 1970. 355 p. [ Links ]
22 Boaventura MF, Fernandez MA, Thiengo SC, Silva RE, Melo AL. Formas larvais de Trematoda provenientes de gastrópodes límnicos da microrregião Rio de Janeiro, sudeste do Brasil. Lundiana. 2002;3(1):45-9. [ Links ]
23 Deslandes N. Técnica de dissecção e exame de planorbídeo s. Rev Serv Espec Saude Publica (Rio de Janeiro). 1951;4(2):371-82. [ Links ]
24 Pan American Health Organization. A guide for the identification of the snail intermediate hosts of schistosomiasis in the Americas. Washington: Pan American Health Organization; 1968. 122 p. (Scientific publication; no.168). [Link] [ Links ]
25 Paraense WL. Estado atual da sistemática dos planorbídeos brasileiros. Arq Mus Nac (Rio de Janeiro). 1975;55:105-28. [ Links ]
26 Barbosa FS. Aspectos da biologia de Zygocotyle lunata (Trematoda: Zygocotylidae) isolado de Biomphalaria straminea (Mollusca: Planorbidae) oriundas de Iguatama, Minas Gerais, Brasil [dissertação]. Belo Horizonte (MG): Universidade Federal de Minas Gerais, Instituto de Ciências Biológicas; 2011. 58 p. [Link] [ Links ]
27 Ximenes RF, Gonçalves ICB, Miyahira IC, Pinto HA, Melo AL, Santos SB. Centrocestus formosanus (Trematoda: Heterophyidae) in Melanoides tuberculata (Gastropoda: Thiaridae) from Vila do Abraão, Ilha Grande, Rio de Janeiro, Brazil. Braz J Biol. 2017 Apr-Jun;77(2):318-22. Doi: 10.1590/1519-6984.13615 [Link] [ Links ]
28 Murillo EAP. Estudo morfológico e molecular de trematódeos transmitidos por Melanoides tuberculata (Mollusca: Thiaridae) em coleções aquáticas do Peru [dissertação]. Belo Horizonte (MG): Universidade Federal de Minas Gerais, Instituto de Ciências Biológicas; 2017. 100 p. [ Links ]
29 Locke SA, McLaughlin JD, Lapierre AR, Johnson PTJ, Marcogliese DJ. Linking larvae and adults of Apharyngostrigea cornu, Hysteromorpha triloba, and Alaria mustelae (Diplostomoidea: Digenea) using molecular data. J Parasitol. 2011 Oct;97(5):846-51. Doi: 10.1645/GE-2775.1 [Link] [ Links ]
30 Silva RE, Melo AL. Caracterização de larvas de trematódeos emergentes de moluscos de água doce coletados na bacia hidrográfica do Lago Soledade, Ouro Branco, Minas Gerais, Brasil. Lundiana. 2013;11(1/2):21-33. [ Links ]
31 Moraes J, Silva MPN, Ohlweiler FP, Kawano T. Schistosoma mansoni and other larval trematodes in Biomphalaria tenagophila (Planorbidae) from Guarulhos, São Paulo State, Brazil. Rev Inst Med Trop S. Paulo. 2009 Mar-Apr;51(2):77-82. Doi: 10.1590/s0036-46652009000200004 [Link] [ Links ]
32 Ruiz JM. Contribuição ao estudo das formas larvárias de trematódeos brasileiros. 2. Fauna de Santos, estado de S. Paulo. Mem Inst Butantan. 1952;24(1):17-36. [Link] [ Links ]
33 Pinto HA, Melo AL. Melanoides tuberculata (Mollusca: Thiaridae) as an intermediate host of Centrocestus formosanus (Trematoda: Heterophyidae) in Brazil. Rev Inst Med Trop S. Paulo. 2010 Jul-Aug;52(4):207-10. Doi: 10.1590/s0036-46652010000400008[Link] [ Links ]
34 Hung NM, Madsen H, Fried B. Global status of fish-borne zoonotic trematodiasis in humans. Acta Parasitol. 2013 Sep;58(3):231-58. Doi: 10.2478/s11686-013-0155-5 [Link] [ Links ]
35 Ohlweiler FP, Eduardo JM, Takahashi FY, Crein GA, Luca LR, Oliveira RC. Larvas de trematódeos associadas a moluscos de água doce em municípios da Região Metropolitana de São Paulo, Estado de São Paulo, Brasil. Rev Pan-Amaz Saude. 2013;4(3):37-48. Doi: 10.5123/S2176-62232013000300006 [Link] [ Links ]
36 Artigas PT, Perez DM, Baggio D. Censo coprológico no município de Peruibe (litoral sul do estado de São Paulo). Registro de casos autóctones de esquistossomose mansoni. Rev Saude Publica (Sao Paulo). 1969 dez;3(2):141-7. [Link] [ Links ]
Received: April 20, 2020; Accepted: February 22, 2021











 text in
text in 


