Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Pan-Amazônica de Saúde
versão impressa ISSN 2176-6215versão On-line ISSN 2176-6223
Rev Pan-Amaz Saude vol.12 Ananindeua 2021 Epub 25-Jun-2021
http://dx.doi.org/10.5123/s2176-6223202100542
ORIGINAL ARTICLE
Streptococcus agalactiae: colonization of high-risk pregnant women in a regional hospital in the Brazilian Amazon and antimicrobial sensitivity profile
2 Faculdade de Ensino Superior da Amazônia Reunida, Redenção, Pará, Brasil
INTRODUCTION:
Group B streptococcus (GBS) or Streptococcus agalactiae in immunosuppressed individuals, such as neonates, can result in a series of complications and diseases, which can even lead to death.
OBJECTIVES:
To characterize the clinical-epidemiological profile of pregnant women colonized by S. agalactiae and determine the isolates' sensitivity profile in a hospital in the Amazon.
MATERIALS AND METHODS:
Clinical specimens were collected from March 15 to April 15, 2019, following the Centers for Disease Control and Prevention guidelines. The phenotypic identification was performed according to the Brazilian Health Regulatory Agency (Anvisa), and for the antimicrobial sensitivity testing, the Clinical and Laboratory Standards Institute specifications were followed. RESULTS: Colonization by GBS was found in 34.0% of the pregnant women; the most frequent chronic diseases were hypertension (26.0%) and diabetes (10.0%). The antimicrobials linezolid, vancomycin, and meropenem were the most effective against the bacteria. There was a high resistance rate for ciprofloxacin (82.4%) and chloramphenicol (70.6%); 88.2% of the strains analyzed were multidrug-resistant.
CONCLUSION:
The presence of GBS among high-risk pregnant women and the detection of multidrug-resistant strains, including those with resistance to penicillins and cephalosporins, bring up the importance of screening for the detection of this bacteria during pregnancy and the beginning of antibiotic prophylaxis, emphasizing the need to adapt the practice of local prenatal care to the current recommendations.
Keywords: Streptococcus agalactiae; Pregnant Women; Microbial Drug Resistance
INTRODUCTION
Streptococcus agalactiae or Lancefield group B Streptococcus (GBS) shows cocci chain morphology and gram-positive dye reaction. It can colonize women's gastrointestinal and genitourinary tracts asymptomatically and cause severe infections in newborns1,2. When the bacterium is transferred to the newborn at the time of delivery, it can cause sepsis and meningitis, increasing the risk of mortality3. The Centers for Disease Control and Prevention (CDC) treats infection/colonization by this bacterium as one of the leading infectious causes of neonatal morbidity and mortality in the United States of America4. A study carried out in Mozambique with a total of 183 stillborns detected GBS in 2.1% of them through umbilical blood analysis5.
Complications during pregnancy, childbirth, and puerperium can be prevented through prenatal care, making it possible to identify risk situations for the mother and fetus6. However, a study conducted with 100 pregnant women to detect colonization by GBS demonstrated a positivity of 14%7. Another study carried out in Rio de Janeiro State revealed that among the 3,647 pregnant women between the 35th and 37th gestational week, 26.2% were colonized by this bacterium8.
Thus, to reduce the associated perinatal damage, Toyofuku et al.9 demonstrated that intrapartum prophylaxis in colonized pregnant women reduced neonatal death by 32.5% in a region with high prevalence rates of this bacterium. The CDC4 recommends screening for GBS colonization in women between the 35th and 37th gestational week and starting antibiotic prophylaxis as soon as the presence of GBS is identified. The Ministry of Health recommends the investigation of these bacteria only in high-risk pregnant women who present signs and symptoms, such as bleeding. However, the pregnant woman may be colonized and have no symptoms and may transmit this microorganism to the fetus, which will be susceptible to infection that can lead to sepsis10.
A high-risk pregnancy is characterized by greater susceptibility to damage to the health of the mother and fetus, such as complications in labor (premature delivery), maternal clinical diseases, and fetal changes that can trigger complications during this period11. Additionally, sociodemographic factors, such as low levels of income, education level, high pregnancy rates, and poor obstetric care favor the persistence of mortality indicators among pregnant women12,13,14.
Besides, bacteria of the genus Streptococcus may present resistance to a prophylactic antimicrobial drug due to chemical changes in the antimicrobial binding sites in the cell wall and transfer of resistance coding genes15. They can also form communities that allow their survival in hostile environments for long periods, called biofilms15. This bacterium is a significant cause of infection in pregnant women and their newborns, and despite this, it has been little studied in Latin America16.
Knowledge about the colonization and sensitivity profile of isolated GBS in pregnant women is of great value, considering the possibility of vertical transmission, its pathogenicity, inherent risks to the health of the mother and fetus, and the possibility of antimicrobial resistance. In addition to these factors, during pregnancy, the administration of antimicrobials is complicated, especially in the case of high-risk pregnant women, and, in Pará State, Brazil, there is a paucity of epidemiological data on this subject.
Given the above, this research aimed to characterize the clinical-epidemiological profile of pregnant women colonized by S. agalactiae and determine the sensitivity profile of these isolates in a hospital that provides medium and high-complexity services in the Amazon.
MATERIALS AND METHODS
ETHICAL ASPECTS
The research was approved by the Comitê de Ética em Pesquisa do Sul do Pará, belonging to the Faculdade de Ensino Superior da Amazônia Reunida, on October 29, 2018, under the number CAAE: 99503018.2.0000.8104; and, in compliance with Resolution No. 466/12 of the National Health Council, the collections were only started in March 2019, after approval by the Committee.
TYPE AND LOCATION OF STUDY
The study was descriptive, prospective, and cross-sectional, with a quantitative approach, carried out in a public hospital, a reference in high-risk pregnancy, which treats an average of 100 pregnant women per month and provides medium and high complexity services to patients from 15 municipalities in Pará State southeast. It belongs to the 12th Regional Health Center, which encompasses a population of 541,347 inhabitants. The hospital is located in Redenção, at a distance of 1,018 km from the capital Belém17,18.
STUDY PERIOD AND POPULATION
The sample collections were performed from March 15 to April 15, 2019, totaling 32 consecutive days. The following inclusion criteria were considered for the study population: women undergoing prenatal care at the hospital with a high-risk pregnancy. Although the CDC4 recommends performing the exam from the 35th gestational week onwards, pregnant women from the 22nd onward were also included in this research. In compliance with the resolution of the National Health Council No. 466/1219, all pregnant women who agreed to participate in the study signed the Free and Informed Consent Form (FICF). Pregnant women who could not sign the FICF were excluded, that is, those who needed the signature of their guardian or curator, as well as those who took antibiotics in the last 10 days.
Thus, 50 pregnant women participated in the study. Two sterile alginate swabs were collected from each of them, one rectal and one vaginal, which were immediately sent for analysis in the hospital's laboratory, following recommendations described later.
Sociodemographic and clinical-obstetric data were analyzed considering the variables: race, education attainment, housing (rural/urban), city of origin, age group, chronic diseases (baseline), sexually transmitted infections (STI), number of pregnancies, and gestational age at the time of swabs collection.
PROCEDURES FOR SAMPLE COLLECTION, PHENOTYPIC DETECTION, AND SENSITIVITY PROFILE
Collections of clinical specimens were performed following CDC recommendations4. Samples were obtained from the vaginal introitus and rectum using sterile alginate swabs. The material was immediately inoculated into Todd Hewitt selective enrichment broth (Probac®) supplemented with antibiotics, hermetically sealed, and taken to the hospital's laboratory.
In the microbiology sector, inoculated samples were incubated at 35-37 °C for 24 h; later, the streaking technique was performed on Todd Hewitt Blood (Probac®), first adding a Hemolysinabac tape (Probac®) in the center of the plate. Streaks perpendicular to the tape were made with a sterile and disposable bacteriological loop, and the plates were reincubated at 35-37 °C for 24 h. The interpretation of the test with detection of β-hemolysis with arrow formation was performed using a positive control for the CAMP test after 24 h. Following the recommendations of the Brazilian Health Regulatory Agency for the phenotypic identification of S. agalactiae20, the strains were submitted to the catalase and PYR (Probac®) tests to determine the pyrrolidonyl arylamidase enzyme activity, in which negativity was observed for all strains included in this research.
The antimicrobial susceptibility test was performed from recent cultures, with sample suspensions in sterile saline solution, with turbidity corresponding to 0.5 of the MacFarland standards in blood agar, by the disk diffusion method described by Clinical and Laboratory Standards Institute (CLSI)21. As a control, a strain of Streptococcus pneumoniae (ATCC® 49619TM) was used. After 24 h of incubation at 35-37 °C, the diameter of the growth inhibition zone was recorded in millimeters, and the isolates were classified as sensitive, intermediate, and resistant, according to CLSI criteria.21. To verify the induced resistance to clindamycin, the D-test was performed, in which the clindamycin disk (Polisensidisc®) has been added to a different site for better viewing.
The antimicrobials tested were penicillin (10 U), ampicillin (10 μg), cefotaxime (30 μg), erythromycin (15 μg), clindamycin (2 μg), chloramphenicol (30 μg), oxacillin (1 μg), azithromycin (15 μg), ciprofloxacin (5 μg), tetracycline (30 μg), linezolid (30 μg), vancomycin (30 μg), and meropenem (10 μg)22. The resistance to three or more classes of antimicrobials tested was defined as resistance to multiple drugs22.
RESULTS
Samples of vaginal and rectal secretions from 50 high-risk pregnant women attended at a regional hospital in the southeast of Pará State were screened for the presence of S. agalactiae. The bacteria was found in 34.0% (17/50) of the pregnant women, of which 35.3% (6/17) in vaginal secretions, 23.5% (4/17) in the anal region, and 41.2% (7/17) in both. The average age of participants was 26.4 years (standard deviation ± 7.2). The highest frequency of positive tests was observed in full-term pregnant women, with ≤ eight years of schooling, non-white skin color, living in rural areas, and who had been pregnant more than once (Table 1).
Table 1 - Sociodemographic profile, clinical-obstetric factors, and frequency of pregnant women colonized by GBS assisted in a regional hospital in Pará State, Brazil, between March and April 2019
| Exposure Variables | Pregnant women | Positive exams | ||
|---|---|---|---|---|
| N = 50 | % | N = 17 | % | |
| Age (X = 26,4; standard deviation ± 7,2) | ||||
| ≤ 25 | 24 | 48,0 | 8 | 33,3 |
| ≥ 26 | 26 | 52,0 | 9 | 34,6 |
| Education attainment | ||||
| ≤ 8 years | 9 | 18,0 | 5 | 55,6 |
| ≥ 9 years | 41 | 82,0 | 12 | 29,3 |
| Skin color | ||||
| White | 4 | 8,0 | 1 | 25,0 |
| Non-white | 46 | 92,0 | 16 | 34,8 |
| Área de residência | ||||
| Rural | 7 | 14,0 | 4 | 57,1 |
| Urban | 43 | 86,0 | 13 | 30,2 |
| Nº of pregnancies | ||||
| Primigravida | 21 | 42,0 | 6 | 28,6 |
| Multigravida | 29 | 58,0 | 11 | 37,9 |
| Gestational age (time of swab collection) | ||||
| (X = 31,9; standard deviation ± 5,9) | ||||
| Preterm < 37 weeks | 35 | 70,0 | 10 | 28,6 |
| Full term from 37 to 42 weeks | 15 | 30,0 | 7 | 46,7 |
| Postterm > 42 weeks | - | - | - | - |
| STI diagnosis (in the current pregnancy) | ||||
| Yes | 3 | 6,0 | - | - |
| No | 47 | 94,0 | 17 | 36,2 |
Conventional sign used: - Numeric data equal to zero, not resulting from rounding.
Among the factors that justify the monitoring of these pregnant women in medium and high-complexity care, the most frequently observed were hypertension (26.0%; 13/50), diabetes (10.0%; 5/50), twin pregnancy (10.0%; 5/50), kidney disease (8.0%; 4/50), and maternal age (8.0%; 4/50). Another associated risk was the STIs, such as syphilis, viral hepatitis B, and HIV infection, which was observed in 6.0% (3/50) of pregnant women.
Regarding the sensitivity profile of the isolated microorganisms, 100.0% (17/17) were sensitive to the antimicrobials linezolid, vancomycin, and meropenem (Figure 1). However, high rates of resistance were detected for ciprofloxacin (82.4%; 14/17), followed by chloramphenicol (70.6%; 12/17), azithromycin (58.8%; 10/17), and tetracycline (58.8%; 10/17). Of the strains analyzed, 88.2% (15/17) were multiresistant, showing resistance to erythromycin, ampicillin, penicillin, ciprofloxacin, and tetracycline (Table 2). The classes of antimicrobials with the highest resistance rates (in vitro) were the quinolones, followed by β-lactams, amphenicols, macrolides, lincosamides, and tetracyclines.
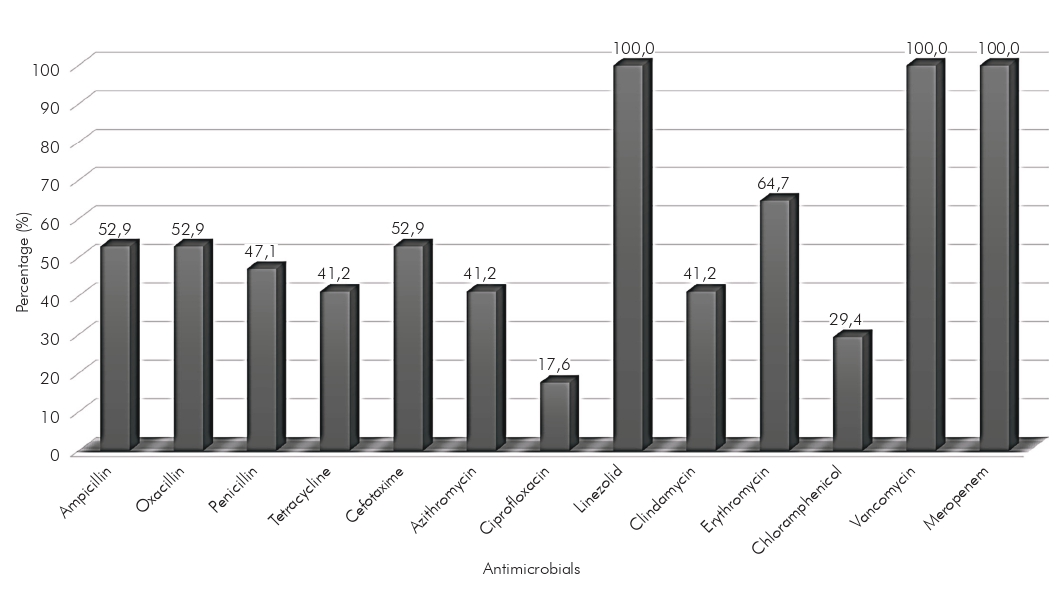
Figure 1 - Sensitivity profile against antimicrobials tested in disk-diffusion (in vitro) of the strains of Streptococcus agalactiae isolated from high-risk pregnant women in southeastern Pará state, Brazil, between March and April 2019
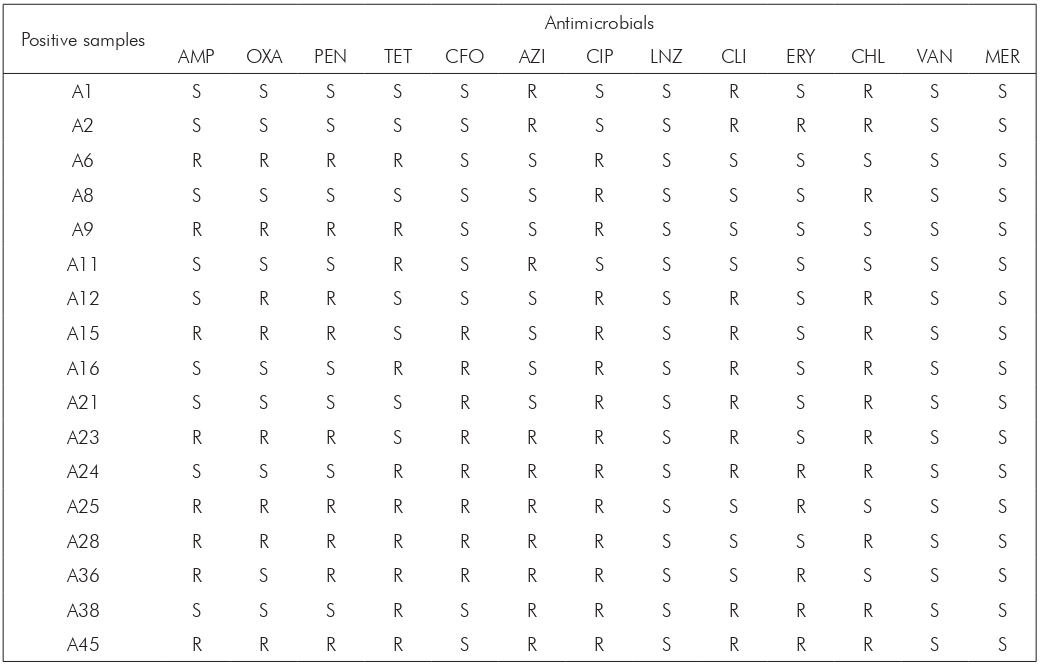
A: Demonstrative data representing positive samples for Streptococcus agalactiae, named according to the identification received during analysis; S: Sensitive; R: Resistant. AMP: Ampicillin; OXA: Oxacillin; PEN: Penicillin; TET: Tetracycline; CFO: Cefotaxime AZI: Azithromycin; CIP: Ciprofloxacin; LNZ: Linezolid; CLI: Clindamycin; ERY: Erythromycin; CHL: Chloramphenicol; VAN: Vancomycin; MER: Meropenem.
Table 2 - Sensitivity profile of Streptococcus agalactiae isolated from 17 high-risk pregnant women in Pará State, Brazil, between March and April 2019
DISCUSSION
In this study, colonization by GBS was found in high-risk pregnant women. This fact can be associated with anatomical and physiological changes, such as hypertrophy of the vaginal walls, increased blood flow, changes in pH, temperature, and vaginal acidity, in addition to some personal hygiene habits such as, for example, the use of showers and intimate soaps23,24.
Although sampling is a limiting factor in this research, studies aimed at analyzing sociodemographic factors can infer the existence of groups more susceptible to colonization by GBS25,26. This fact was demonstrated in a study conducted in Saudi Arabia with 1,328 pregnant women, in which colonization by GBS was 4.2% in pregnant women aged between 25 and 29 years, and 27.4% among those aged over 40 years27.
The present research results agree with those of a study carried out in Rio de Janeiro, which demonstrated that arterial hypertension and gestational diabetes were the most observed maternal pathologies among high-risk pregnant women8. The pathology that most predisposes to maternal colonization is still not well understood; however, it is assumed that the change in immune status, as occurs in diabetes during pregnancy, enables invasive GBS infection28,29,30.
Drugs used for the treatment of GBS must have the ability to inhibit cell wall synthesis, protein synthesis, and DNA gyrase. However, some antimicrobials cannot be prescribed during pregnancy because they could break the placental barrier and harm the health of the mother and fetus. Thus, penicillin, amoxicillin, ampicillin, cephalexin, and nitrofurantoin are recommended during pregnancy and, in case of allergies to penicillin, clindamycin or erythromycin4,31.
However, when analyzing the effectiveness of these antimicrobials for the isolates in this research, it was possible to observe that the penicillins and cephalosporins tested did not show good sensitivity (in vitro). Corroborating this data, a study conducted in Ceará State showed that among isolated GBS strains, some were resistant to ampicillin (44.4%), cephalotin (44.4%), clindamycin (77.8%), chloramphenicol (11, 1%), erythromycin (33.3%), and penicillin (44.4%)32. Low rates of resistance of this bacterium to erythromycin had been reported in other studies, revealing sensitivity rates for this drug of 85.7% and 92.5%33,34.
Indices of reduced sensitivity of GBS to penicillin have been described in the international literature35,36. These indices can be associated with several factors, such as its large-scale use and mutations that alter the binding site of penicillin binding proteins; those mutations can be carried by plasmids and transferred horizontally between different species35,36. However, the description of GBS resistance to this drug in Brazil remains very low, and this may be related to the low rates of research focused on this subject and the lack of structure to carry out the test16,32. As in this research, Linhares et al.32 reported that the GBS test for pregnant women was not performed in prenatal routines.
In addition, as demonstrated in a study carried out in Amazonas State, penicillins and cephalosporins are among the main drugs used, especially cephalexin (39.7%), amoxicillin (29.4%), followed by benzathine benzylpenicillin (4.4%), ciprofloxacin (3.7%), sulfadiazine (3.7%), tetracycline (3.7%), azithromycin (2.9%) and levofloxacin (2.9%), which may influence the increased detections of strains resistant to these drugs37.
The bacteria analyzed in this research showed high levels of resistance to the quinolone tested. According to research carried out in Argentina, this fact is associated with point mutations in gyrA and parC genes38. The importance of monitoring the sensitivity of this bacterium is highlighted, as, in addition to presenting virulence factors that facilitate the development of an infectious process, it may present resistance mechanisms that hinder the therapeutic approach38,39.
The indiscriminate use of medications can promote the emergence of multidrug-resistant bacteria associated with high morbidity and mortality40,41. With the advent of bacterial resistance, the identification of the pathogen and knowledge about its sensitivity profile in infection/colonization during pregnancy became important for efficient intrapartum prophylaxis. In this regard, the CDC recommends screening based on microbiological results, with detection of colonization by swab collection routinely performed between the 35th and 37th gestational week4,41.
Similarly, the American College of Obstetricians and Gynecologists recommends performing GBS screening between the 36th and 37th week of pregnancy, with appropriate intrapartum antibiotic prophylaxis42. However, in this study, pregnant women from the 22nd gestational week were considered, considering the risk of premature birth, as they are high-risk pregnant women; the fact that colonization by GBS during pregnancy is transient, intermittent, or constant; and that, in the hospital under study, the test for the presence of GBS is not carried out at any stage of pregnancy. Thus, it is noteworthy that there is scientific evidence that suggests the association between colonization by this bacterium and prematurity, resulting in increased mortality rates43,44.
In the hospital where the present study was carried out, despite specifically attending high-risk pregnant women, the exam for the detection of GBS is not performed as part of prenatal care, which can increase the risks to the health of the mother and fetus. On the other hand, in a hospital in São Paulo State, preventive strategies were implemented considering correct periods for collection, higher positivity rates, and risks inherent to the health of the mother and fetus for screening and detecting GBS, making it possible to mitigate the number of infections from 57.8% to 55.1%, which shows the importance of public policies and strategies to improve health care44,45.
It is also important to emphasize that the interpretation of the results was made in light of limitations. The number of participants was small, thus limiting the possibility of representativeness of resistance detections. This may be associated with the fact that the hospital only attends high-risk pregnant women.
CONCLUSION
It was possible to evidence the presence of GBS among high-risk pregnant women attended at a regional hospital in a city in southeastern Pará and to detect multidrug-resistant strains, including those with resistance to penicillins and cephalosporins. The frequencies of positivity for colonization by GBS and of multidrug resistance detected highlight the importance of screening for microbiological detection of this bacterium during pregnancy and the beginning of antibiotic prophylaxis when indicated, demonstrating the need to adapt prenatal care practice to current recommendations. Knowledge about the clinical-epidemiological profile described in this study can contribute to the development of local preventive measures.
It is also recommended to conduct further studies with a larger sample size and an epidemiological characterization following current recommendations to correlate colonization by maternal GBS with damage to the fetus's health.
ACKNOWLEDGMENTS
To the Faculdade de Ensino Superior da Amazônia Reunida and its teaching staff, for providing the necessary conditions to reach the goals.
REFERENCES
1 Melo SCCS, Santos NCS, Oliveira M, Scodro RBL, Cardoso RF, Pádua RAF, et al. Antimicrobial susceptibility of Streptococcus agalactiae isolated from pregnant women. Rev Inst Med Trop Sao Paulo. 2016 Nov;58:83. Doi: 10.1590/S1678-9946201658083 [Link] [ Links ]
2 Tortora GJ, Funke BR, Case CL. Microbiologia. 10. ed. Porto Alegre: Artmed; 2012. Capítulo 11, Procariotos: domínios Bacteria e Archaea; p. 299-328. [ Links ]
3 Choi SY, Kim JW, Ko JW, Lee YS, Chang YP. Patterns of ischemic injury on brain images in neonatal group B Streptococcal meningitis. Korean J Pediatr. 2018 Aug;61(8):245-52. Doi: 10.3345/kjp.2018.61.8.245 [Link] [ Links ]
4 Centers for Disease Control and Prevention. Prevention of perinatal group B streptococcal disease: revised guidelines from CDC, 2010 [Internet]. Atlanta: Centers for Disease Control and Prevention; 2019 [cited 2019 Jan 12]. Available from: Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5910a1.htm . [ Links ]
5 Sigaúque B, Kobayashi M, Vubil D, Nhacolo A, Chaúque A, Moaine B, et al. Invasive bacterial disease trends and characterization of group B streptococcal isolates among young infants in southern Mozambique, 2001-2015. PLoS One. 2018 Jan;13(1):e0191193. Doi: 10.1371/journal.pone.0191193 [Link] [ Links ]
6 Ministério da Saúde (BR). Secretaria de Atenção à Saúde. Departamento de Ações Programáticas Estratégicas. Gestação de alto risco: manual técnico. 5. ed. Brasília: Ministério da Saúde; 2010. (Série A. Normas e manuais técnicos). [Link] [ Links ]
7 Nkembe NM, Kamga HG, Baiye WA, Chafa AB, Njotang PN. Streptococcus agalactiae prevalence and antimicrobial susceptibility pattern in vaginal and anorectal swabs of pregnant women at a tertiary hospital in Cameroon. BMC Res Notes. 2018 Jul;11(1):480. Doi: 10.1186/s13104-018-3589-x [Link] [ Links ]
8 Botelho ACN, Oliveira JG, Damasco AP, Santos KTB, Ferreira AFM, Rocha GT, et al. Streptococcus agalactiae carriage among pregnant women living in Rio de Janeiro, Brazil, over a period of eight years. PLoS One. 2018 May;13(5):e0196925. Doi: 10.1371/journal.pone.0196925 [Link] [ Links ]
9 Toyofuku M, Morozumi M, Hida M, Satoh Y, Sakata H, Shiro H, et al. Effects of intrapartum antibiotic prophylaxis on neonatal acquisition of group B streptococci. J Pediatr. 2017 Nov;190:169-73.e1. Doi: 10.1016/j.jpeds.2017.07.039 [Link] [ Links ]
10 Ministério da Saúde (BR). Secretaria de Atenção à Saúde. Departamento de Atenção Básica. Atenção ao pré-natal de baixo risco. Brasília: Ministério da Saúde; 2012. (Série A. Normas e manuais técnicos. Cadernos de atenção básica; n. 32). [Link] [ Links ]
11 Costa ALRR, Araújo Jr E, Lima JWO, Costa FS. Maternal risk factors associated with the necessity of neonatal intensive care unit. Rev Bras Ginecol Obstet. 2014 Jan;36(1):29-34. [Link] [ Links ]
12 Hogan MC, Foreman KJ, Naghavi M, Ahn SY, Wang M, Makela SM, et al. Maternal mortality for 181 countries, 1980-2008: a systematic analysis of progress towards Millennium Development Goal 5. Lancet. 2010 May:375(9726):1609-23. Doi: 10.1016/S0140-6736(10)60518-1 [Link] [ Links ]
13 Le Doare K, O'Driscoll M, Turner K, Seedat F, Russell NJ, Seale AC, et al. Intrapartum antibiotic chemoprophylaxis policies for the prevention of group B streptococcal disease worldwide: systematic review. Clin Infect Dis. 2017 Nov;65 Suppl 2:S143-51. Doi: 10.1093/cid/cix654 [Link] [ Links ]
14 Heath PT, Jardine LA. Neonatal infections: group B streptococcus. BMJ Clin Evid. 2014 Feb;2014:0323. [Link] [ Links ]
15 Rosini R, Margarit I. Biofilm formation by Streptococcus agalactiae: influence of environmental conditions and implicated virulence factors. Front Cell Infect Microbiol. 2015 Feb;5:6. Doi: 10.3389/fcimb.2015.00006 [Link] [ Links ]
16 Zusman AS, Baltimore RS, Fonseca SNS. Prevalence of maternal group B streptococcal colonization and related risk factors in a Brazilian population. Braz J Infect Dis. 2006 Aug;10(4):242-6. Doi: 10.1590/s1413-86702006000400005 [Link] [ Links ]
17 Instituto Brasileiro de Geografia e Estatística. Redenção [Internet]. Rio de Janeiro: IBGE; 2017 [citado 2019 set 3]. Disponível em: Disponível em: https://cidades.ibge.gov.br/brasil/pa/redencao/panorama . [ Links ]
18 Pará. Secretaria de Saúde Pública. Relatório anual de gestão do 12º Centro Regional de Saúde/SESPA referente ao exercício 2016. Belém: Secretaria de Saúde Pública; 2017. [Link] [ Links ]
19 Brasil. Ministério da Saúde. Conselho Nacional de Saúde. Resolução nº 466, de 12 de dezembro de 2012. Estabelece diretrizes e normas regulamentadoras de pesquisas envolvendo seres humanos. Diário Oficial da União, Brasília (DF), 2013 jun 13; Seção 1:59. [ Links ]
20 Agência Nacional de Vigilância Sanitária (BR). Gram-positivos: Streptococcus spp [Internet]. Brasília: Agência Nacional de Vigilância Sanitária; 2008 [citado 2018 set 3]. Disponível em: Disponível em: http://www.anvisa.gov.br/servicosaude/controle/rede_rm/cursos/boas_praticas/modulo4/intr_stre.htm . [ Links ]
21 Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. CLSI supplement M100. 29th ed. Wayne (PA): Clinical and Laboratory Standards Institute; 2019. [Link] [ Links ]
22 Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012 Mar;18(3):268-81. Doi: 10.1111/j.1469-0691.2011.03570.x [Link] [ Links ]
23 Lima TM, Teles LMR, Oliveira AS, Campos FC, Barbosa RCC, Pinheiro AKB, et al. Corrimentos vaginais em gestantes: comparação da abordagem sindrômica com exames da prática clínica da enfermagem. Rev Esc Enferm USP. 2013 dez;47(6):1265-71. Doi: 10.1590/S0080-623420130000600002 [Link] [ Links ]
24 Silva AK, Silva ADAT, Barros IM, Lima LR. Vulvovaginites durante a gestação e a importância do tratamento imediato - uma revisão de literatura. In: Anais do 12º Encontro de Extensão, Docência e Iniciação Científica; 2016 dez; Quixadá, CE. Quixadá: Centro Universitário Católica de Quixadá; 2016. [Link] [ Links ]
25 Manning SD, Lewis MA, Springman AC, Lehotzky E, Whittam TS, Davies HD. Genotypic diversity and serotype distribution of group b streptococcus isolated from women before and after delivery. Clin Infect Dis. 2008 Jun;46(12):1829-37. Doi: 10.1086/588296 [Link] [ Links ]
26 Edwards MS, Rench MA, Palazzi DL, Baker CJ. Group B streptococcal colonization and serotype-specific immunity in healthy elderly persons. Clin Infect Dis. 2005 Feb;40(3):352-7. Doi: 10.1086/426820 [Link] [ Links ]
27 Khan MA, Faiz A, Ashshi AM. Maternal colonization of group B streptococcus: prevalence, associated factors and antimicrobial resistance. Ann Saudi Med. 2015 Nov-Dec;35(6):423-7. Doi: 10.5144/0256-4947.2015.423 [Link] [ Links ]
28 Batista RP, Ferreira CR. Streptococcus agalactiae septicemia in a patient with diabetes and hepatic cirrhosis. Autops Case Rep. 2015 Dec;5(4):35-43. Doi: 10.4322/acr.2015.028 [Link] [ Links ]
29 Wang YH, Su LH, Hou JN, Yang TH, Lin TY, Chu C, et al. Group B streptococcal disease in nonpregnant patients: emergence of highly resistant strains of serotype Ib in Taiwan in 2006 to 2008. J Clin Microbiol. 2010 Jul;48(7):2571-4. Doi: 10.1128/JCM.00810-10 [Link] [ Links ]
30 Pitts SI, Maruthur NM, Langley GE, Pondo T, Shutt KA, Hollick R, et al. Obesity, diabetes, and the risk of invasive group B streptococcal disease in nonpregnant adults in the United States. Open Forum Infect Dis. 2018 Jun;5(6):ofy030. Doi: 10.1093/ofid/ofy030 [Link] [ Links ]
31 Silva P. Farmacologia. 8. ed. Rio de Janeiro: Guanabara Koogan; 2010. Seção 9, Antibióticos e quimioterápicos. Venenos animais, conceitos básicos da antibioticoterapia; p. 933-1005. [ Links ]
32 Linhares JJ, Cavalcante Neto PG, Vasconcelos JLM, Saraiva TV, Ribeiro AMF, Siqueira TM, et al. Prevalência de colonização por Streptococcus agalactiae em gestantes atendidas em maternidade do Ceará, no Brasil, correlacionando com os resultados perinatais. Rev Bras Ginecol Obstet. 2011 dez;33(12):395-400. Doi: 10.1590/S0100-72032011001200004 [Link] [ Links ]
33 Numanović F, Smajlović J, Gegić M, Delibegović Z, Bektaš S, Halilović E, et al. Presence and resistance of Streptococcus agalactiae in vaginal specimens of pregnant and adult non-pregnant women and association with other aerobic bacteria. Med Glas (Zenica). 2017 Feb;14(1):98-105. Doi: 10.17392/876-16 [Link] [ Links ]
34 Assefa S, Desta K, Lema T. Group B streptococci vaginal colonization and drug susceptibility pattern among pregnant women attending in selected public antenatal care centers in Addis Ababa, Ethiopia. BMC Pregnancy Childbirth. 2018 May;18(1):135. [ Links ]
35 Kimura K, Suzuki S, Wachino J, Kurokawa H, Yamane K, Shibata N, et al. First molecular characterization of group B streptococci with reduced penicillin susceptibility. Antimicrob Agents Chemother. 2008 Aug;52(8):2890-7. Doi: 10.1128/AAC.00185-08 [Link] [ Links ]
36 Seki T, Kimura K, Reid ME, Miyazaki A, Banno H, Jin W, et al. High isolation rate of MDR group B streptococci with reduced penicillin susceptibility in Japan. J Antimicrob Chemother. 2015 Oct;70(10):2725-8. Doi: 10.1093/jac/dkv203 [Link] [ Links ]
37 Pereira JQ, Silva MT, Galvão TF. Use of antibiotics by adults: a population-based cross-sectional study. Sao Paulo Med J. 2018 Sep-Oct;136(5):407-13. Doi: 10.1590/1516-3180.2018.0168060818 [Link] [ Links ]
38 Arias B, Kovacec V, Vigliarolo L, Suárez M, Tersigni C, Müller L, et al. Fluoroquinolone-resistant Streptococcus agalactiae invasive isolates recovered in Argentina. Microb Drug Resist. 2019 Jun;25(5):739-43. Doi: 10.1089/mdr.2018.0246 [Link] [ Links ]
39 Bolukaoto JY, Monyama CM, Chukwu MO, Lekala SM, Nchabeleng M, Maloba MRB, et al. Antibiotic resistance of Streptococcus agalactiae isolated from pregnant women in Garankuwa, South Africa. BMC Res Notes. 2015 Aug;8:364. Doi: 10.1186/s13104-015-1328-0 [Link] [ Links ]
40 Malheiro LF, Magano R, Ferreira A, Sarmento A, Santos L. Skin and soft tissue infections in the intensive care unit: a retrospective study in a tertiary care center. Rev Bras Ter Intensiva. 2017 Apr-Jun;29(2):195-205. Doi: 10.5935/0103-507X.20170019 [Link] [ Links ]
41 Fiolo K, Zanardi CE, Salvadego M, Bertuzzo CS, Amaral E, Calil R, et al. Taxa de infecção e sorotipos de Streptococcus agalactiae em amostras de recém-nascidos infectados na cidade de Campinas (SP), Brasil. Rev Bras Ginecol Obstet. 2012 dez;34(12):544-9. Doi: 10.1590/s0100-72032012001200003 [Link] [ Links ]
42 Prevention of group B streptococcal early-onset disease in newborns: ACOG committee opinion, number 797. Obstet Gynecol. 2020 Feb;135(2):e51-72. Doi: 10.1097/AOG.0000000000003668 [Link] [ Links ]
43 Bianchi-Jassir F, Seale AC, Kohli-Lynch M, Lawn JE, Baker CJ, Bartlett L, et al. Preterm birth associated with group B streptococcus maternal colonization worldwide: systematic review and meta-analyses. Clin Infect Dis . 2017 Nov;65 Suppl 2:S133-42. Doi: 10.1093/cid/cix661 [Link] [ Links ]
44 Pintye J, Saltzman B, Wolf E, Crowell CS. Risk factors for late-onset group B streptococcal disease before and after implementation of universal screening and intrapartum antibiotic prophylaxis. J Pediatric Infect Dis Soc. 2016 Dec;5(4):431-8. Doi: 10.1093/jpids/piv067 [Link] [ Links ]
45 Prefeitura de São Paulo. Secretaria Municipal de Saúde. Coordenação de Epidemiologia e Informação. Alguns aspectos da evolução da mortalidade infantil na cidade de São Paulo. São Paulo: PMSP/SMS/CEInfo; 2009. [Link] [ Links ]
How to cite this article / Como citar este artigo: Ribeiro EA, Tomich GM, Costa BA, Oliveira RA, Jesus LKB. Streptococcus agalactiae: colonization of high-risk pregnant women in a regional hospital in the Brazilian Amazon and antimicrobial sensitivity profile. Rev Pan Amaz Saude. 2021;12:e202100542. Doi: http://dx.doi.org/10.5123/S2176-6223202100542
Received: November 13, 2019; Accepted: February 26, 2021











 texto em
texto em 


