Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Pan-Amazônica de Saúde
Print version ISSN 2176-6215On-line version ISSN 2176-6223
Rev Pan-Amaz Saude vol.12 Ananindeua 2021 Epub Oct 05, 2021
http://dx.doi.org/10.5123/s2176-6223202100862
ORIGINAL ARTICLE
Comparative epidemiological analysis between pandemics caused by the Influenza A(H1N1)pdm09 and SARS-CoV-2 viruses in Pará State, Brazil
1 Universidade Federal do Pará, Faculdade de Medicina, Belém, Pará, Brasil
2 Instituto Optométrico de Pernambuco, Faculdade de Saúde de Paulista, Ciências Biológicas, Paulista, Pernambuco, Brasil
3 Universidade Federal do Rio Grande do Sul, Programa de Pós-Graduação em Genética e Biologia Molecular, Porto Alegre, Rio Grande do Sul, Brasil
4 Universidade Federal do Pará, Instituto de Ciências Biológicas, Belém, Pará, Brasil
OBJECTIVE:
To analyze the epidemiological characteristics, similarities, and differences between influenza A(H1N1)pdm09 and COVID-19 in Pará State, Brazil.
MATERIALS AND METHODS:
This is a descriptive epidemiological study using data on cases and deaths of influenza A(H1N1)pdm09 from the Notifiable Diseases Information System (SINAN), from June 2009 to May 2010; and COVID-19, from the Health Department of State Pará (SESPA), from March to July 2020. The data were collected on July 10, 2020.
RESULTS:
At the time of data collection, Pará State had 124,934 confirmed cases of COVID-19, while the influenza pandemic had 783. Individuals between 30 and 39 years old (24.95%) were the most affected by COVID-19, with a higher death rate in the elderly (74.00%). On the other hand, the Influenza A(H1N1)pdm09 virus affected more children and young people from 10 to 19 years (31.42%), with higher mortality between 20 and 29 years (26.83%). COVID-19 had a more dispersed distribution of cases in the state compared to the influenza A(H1N1)pdm09 pandemic.
CONCLUSION:
These findings show that the reported scenario on the influenza A(H1N1)pdm09 pandemic in Pará reflects the need to modify the strategic planning that must be implemented in the face of the COVID-19 pandemic, considering its incidence in different people and their pathophysiological differences.
Keywords: COVID-19; Influenza A; SARS-CoV-2; Influenza A Virus, H1N1 Subtype; Pandemic; Epidemiology
INTRODUCTION
With the advancement of epidemiology and understanding of infectious diseases, social relations, demography, and mobility resulting from the globalization process are increasingly becoming protagonists in the dissemination and transmissibility of microorganisms with the potential to be infectious in humans1. According to the World Health Organization (WHO), the term pandemic refers to a state caused by the emergence and dissemination of a new pathogen, with the ability to trigger a disease, often severe, with significant ease, by its replication and transmissibility between humans2,3.
Among the pandemics that have plagued humanity in the last 100 years, those caused by influenza viruses belonging to the Orthomyxoviridae family stand out. Three virus genera (A, B, and C) involved in respiratory conditions belong to this family. The genera A and B cause higher mortality; however, only genus A has pandemic potential. Viruses of the genus Alphaorthomyxovirus (genus A) are classified according to the antigenic subtype of two surface structures, hemagglutinin (H, 19 subtypes) and neuraminidase (N, 11 subtypes), responsible for the processes of viral adsorption and cell receptor cleavage, respectively. In the A H1N1 virus, antigenic subtype 1 of structure H and antigenic subtype 1 of structure N are present. Because it has animal reservoirs and has high virulence in humans, this subtype and its mutations still affect the population today1,4.
Between 1918 and 1920, Influenza A (H1N1), of avian origin, became responsible for the so-called Spanish flu, commonly cited as "the greatest medical holocaust in history", with millions of deaths worldwide5,6. In later years, the constant remodeling and antigenic mutability of the virus brought about new pandemic scenarios. In 1957, a new strain of Influenza (H2N2) caused epidemic outbreaks in several regions of the globe, becoming known as Asian flu7. The H3N2 strain recreated, in 1968, the same scenario with the Hong Kong flu8; and, finally, in 2009, the A(H1N1)pdm09 strain, of swine-origin, had its first cases notified in Mexico, being disseminated, soon after, throughout the European continent and Oceania9. Samples from previous pandemics, such as Spanish flu, English flu, Hong Kong flu, and the Influenza A(H1N1)pdm09 virus, originated from shift-type mutations that occur by viral genome segments recombination of viral samples from different animal origins10.
In an analysis of the severity of the last pandemic brought about by the Influenza A(H1N1)pdm09 variant, according to the survey carried out by the WHO, around 150,000 deaths were registered on the planet until the 1st of April 2010. In Brazil, there had been about 40,000 cases of severe acute respiratory syndrome (SARS) and approximately 1,700 deaths in the eight-month period since the declaration of Public Health Emergency of International Concern (PHEIC) by the WHO in April 200911. The severity of the pandemic, although with medical and scientific resources superior to previous scenarios, revealed the need and urgency for a new approach to epidemiological surveillance and, as a basic requirement, greater efficiency in the detection and monitoring of new cases12.
After five PHEIC declarations (H1N1 in 2009, poliomyelitis in 2014, ebola in 2014 and 2018, and zika in 2016), an outbreak in late 2019 related to the emergence of SARS cases in Wuhan, capital of Hubei Province, China, was caused by a new etiological agent that, in a short time, became the target of the sixth emergency declared by the WHO, showing its potential as a threat to global health: the so-called new coronavirus (SARS-CoV-2)13,14.
The world had already faced a pandemic and an epidemic due to infection by the SARS-CoV and MERS-CoV coronaviruses, both originating in animal reservoirs, of the genus Betacoronavirus and subgenus Sarbecovirus. Between 2002 and 2003, the first was caused by SARS-CoV, thus identified as having caused SARS in infected individuals; the second, caused by MERS-CoV, responsible for the Middle East respiratory syndrome (MERS) in 2011. Finally, emerging, in late 2019, a pandemic caused by another coronavirus, also related to respiratory diseases, with high pathogenicity13.
Based on the similarities and contrasts between the aforementioned pandemics, the present study aims to analyze the epidemiological manifestation of infection by Influenza A(H1N1)pdm09 and SARS-CoV-2 in Pará State, Brazil, in hopes that it may guide the decision-making of health managers in future similar situations.
MATERIALS AND METHODS
This is a descriptive epidemiological study, in which a comparison was made between the epidemiological analysis of influenza caused by the Influenza A(H1N1)pdm09 virus, based on data from the Notifiable Diseases Information System (SINAN), tabulated by the Department of Informatics of the Brazilian Unified Health System (DATASUS), for the period from June 2009 to May 2010. For COVID-19 pandemic, caused by SARS-CoV-2, the data were extracted from the Epidemiological Bulletins of the Secretariat of Health of Pará State (SESPA) for the period from March to July 2020. For both platforms, the last data collection date was July 10, 2020, at 6:01 pm.
An epidemiological study involves the delimitation of variables associated with diseases in a given population sample and in a specific period to verify the association between the variables, characteristic of an observational approach in which the researcher uses data already available and does not directly interfere in research. For the categorization of the information obtained in the analysis of the collected data, the following variables were selected: age, gender, symptoms, number of cases and deaths, mortality rate, hospitalizations due to SARS, final classification, and microregions of notification.
The data obtained were tabulated and analyzed in Microsoft Excel, and graphs, maps, and tables were generated. The maps were elaborated and designed using the Qgis program, where the representation of the incidence of cases directly reflects on the chosen color tone. The creation of the table, the statistical analysis of the incidence and fatality rates, and the calculations aimed at establishing confidence intervals and percentage analyses were performed using the SigmaPlot v12.0 tool.
Finally, the inclusion criteria established was a positive diagnosis for one of the two pathologies. As this research used secondary public data, the Free and Informed Consent Form was not required, as there is no individual identification.
RESULTS
Considering the beginning of notifications of COVID-19 cases in early March 2020, 136,184 tests were carried out in Pará until the date of data collection, of which 124,934 had diagnostic confirmation, 10,900 were discarded, and 350 were still under analysis - numbers that made Pará reach the 5th position among the Brazilian states with the highest number of cases. Of the total number of infected, 109,699 recovered, 9,961 were still hospitalized or in voluntary quarantine, and 5,274 progressed to death, generating a mortality rate of 4.22%.
As for influenza A(H1N1)pdm09, Brazil reached 54,171 confirmed cases between 2009 and 2010, and Pará were the 6th in the ranking nation of infected. A total of 2,184 tests were carried out in the State, with 783 confirmed, 1,265 discarded, and 136 under investigation. Among the confirmed cases, 690 were cured, and 41 progressed to death, with a fatality rate of 5.23%, and 52 were deaths that did not occur directly by influenza, but by other causes, according to DATASUS. In a temporal analysis of the incidence of cases of the two pandemics in the State, it can be observed, in figure 1, the noticeable peak, common to both pandemics, in the third month after the notification of the first case, with 61,300 new cases of COVID-19 and 147 cases of influenza A(H1N1)pdm09. It is also noteworthy that, in relation to notifications of influenza A(H1N1)pdm09, the second wave of notifications was recorded in the second semester.
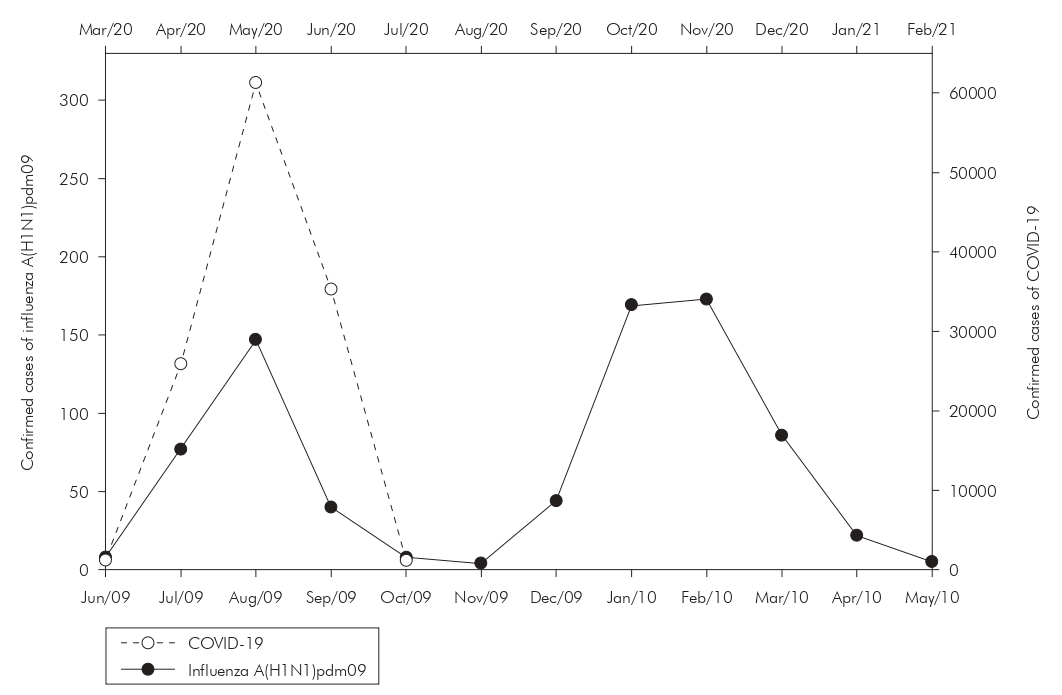
Source: SESPA (COVID-19) and SINAN-DATASUS (influenza A(H1N1)pdm09), July/2020.
* The initial month of analysis corresponds to when the first confirmed case of the pathologies analyzed in the State was notified. The month of June 2009 is for influenza A(H1N1)pdm09 and March 2020 for COVID-19. The information for the month of July 2020 corresponds to the data available until July 10, 2020.
Figure 1 - Distribution of confirmed cases of COVID-19 and influenza A(H1N1)pdm09 over the months* in Pará State, Brazil
As for mortality, in addition to the substantial difference in the number of absolute deaths, there was a less abrupt drop in deaths from influenza A(H1N1)pdm09 than that observed from the fourth month of the COVID-19 pandemic, associated with a second wave of influenza A(H1N1)pdm09, greater and longer-lasting, reaching, in the eighth month, the peak of new deaths, as seen in figure 2.
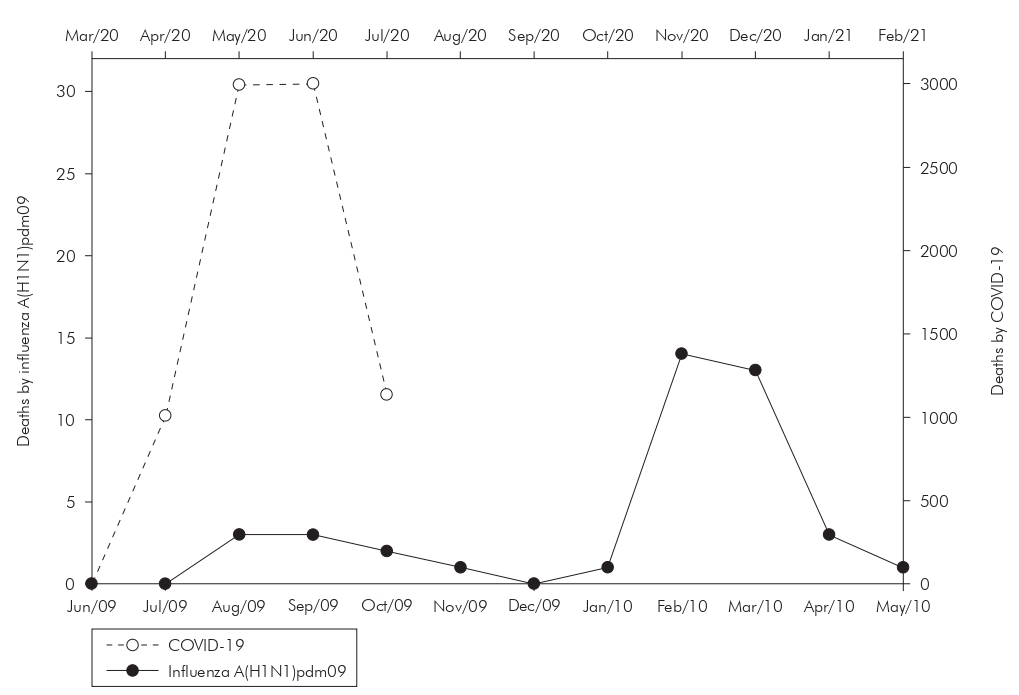
Source: SESPA (COVID-19) and SINAN-DATASUS (influenza A(H1N1)pdm09), July/2020.
* The initial month of analysis corresponds to when the first confirmed case of the pathologies analyzed in the State was notified. The month of June 2009 is for influenza A(H1N1)pdm09 and March 2020 for COVID-19.The information for the month of July 2020 corresponds to the data available until July 10, 2020.
Figure 2 - Distribution of new deaths caused by COVID-19 and influenza A(H1N1)pdm09 over the months* in Pará State, Brazil
While SARS-CoV-2 affected more adults in the 30 to 39 year age group, the Influenza A(H1N1)pdm09 virus affected more children and young people from 10 to 19 years. Regarding the incidence by gender of those affected by COVID-19, there was an equivalence of virus infection in the State, with 60,525 (48.45%) male and 64,409 (51.55%) female. Regarding influenza A(H1N1)pdm09, the number of infected women was higher, totaling 477 (60.92%), and men was 306 (39.08%) (Table 1).
Table 1 - Number of confirmed cases and deaths from COVID-19 (from March to July 2020) and from influenza A(H1N1)pdm09 (from June 2009 to May 2010), by age group and sex, in Pará State, Brazil
| Confirmed cases | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variables | COVID-19 | Influenza A(H1N1)pdm09 | ||||||
| N | % | CI 95% | N | % | CI 95% | |||
| Age group (years) | ||||||||
| 0 a 9 | 3.675 | 2,94 | 0,91-4,96 | 187 | 23,88 | 22,34-25,41 | ||
| 10 a 19 | 6.265 | 5,01 | 0,36-9,65 | 246 | 31,42 | 29,38-33,43 | ||
| 20 a 29 | 19.502 | 15,61 | 13,24-17,97 | 156 | 19,92 | 17,73-22,10 | ||
| 30 a 39 | 31.173 | 24,95 | 24,09-25,80 | 80 | 10,22 | 8,78-11,63 | ||
| 40 a 49 | 25.831 | 20,68 | 19,15-22,20 | 44 | 5,62 | 5,27-5,94 | ||
| 50 a 59 | 17.113 | 13,70 | 12,77-14,62 | 38 | 4,85 | 4,47-5,22 | ||
| ≥ 60 | 21.375 | 17,11 | 16,29-17,92 | 32 | 4,09 | 3,70-4,45 | ||
| Gender | ||||||||
| Male | 60.525 | 48,45 | 48,00-48,87 | 306 | 39,08 | 35,65-42,50 | ||
| Female | 64.409 | 51,55 | 51,12-51,97 | 477 | 60,92 | 58,14-63,67 | ||
| Confirmed deaths | ||||||||
| Variables | COVID-19 | Influenza A(H1N1)pdm09 | ||||||
| N | % | CI 95% | N | % | CI 95% | |||
| Age group (years) | ||||||||
| 0 a 9 | 29 | 0,55 | 0,45-0,68 | 5 | 12,20 | 10,13-14,26 | ||
| 10 a 19 | 23 | 0,44 | 0,33-0,56 | 8 | 19,51 | 17,86-21,15 | ||
| 20 a 29 | 61 | 1,16 | 0,55-1,84 | 11 | 26,83 | 23,54-30,11 | ||
| 30 a 39 | 190 | 3,60 | 1,39-6,08 | 4 | 9,75 | 8,96-10,55 | ||
| 40 a 49 | 430 | 8,15 | 7,09-8,66 | 5 | 12,20 | 11,50-12,89 | ||
| 50 a 59 | 638 | 12,10 | 9,34-10,67 | 5 | 12,20 | 11,50-12,89 | ||
| ≥ 60 | 3.903 | 74,00 | 68,69-83,53 | 3 | 7,31 | 5,51-9,12 | ||
| Gender | ||||||||
| Male | 3.365 | 63,80 | 60,39-67,20 | 10 | 24,39 | 14,14-34,63 | ||
| Female | 1.909 | 36,20 | 31,68-40,69 | 31 | 75,61 | 69,80-81,39 | ||
Source: SESPA (COVID-19) and SINAN-DATASUS (influenza A(H1N1)pdm09), July/2020.
N: Total number; CI: Confidence interval 95%.
In addition, the death rate from COVID-19 was predominant among men, with 63.80% (3,365), while among women, it was 36.20% (1,909). For influenza A(H1N1)pdm09, the number of deaths reported was 31 (75.61%) female and 10 (24.39%) male. While infection with the Influenza A(H1N1)pdm09 virus promoted higher fatality among individuals under 30 years of age, SARS-CoV-2 reached the elderly.
According to the symptoms presented, influenza A(H1N1)pdm09 syndromes were identified in 2009: flu syndrome, characterized by at least two of the signs and symptoms (fever, chills, sore throat, headache, cough, runny nose, olfactory disorders, or taste disorders), in 3.07% of cases; SARS, having the same symptoms as the flu syndrome plus dyspnea or respiratory distress, oxygen saturation lower than 95% in room air, and cyanosis, in 60% of cases; and SARS with hospitalization, in 79.43% of the reported cases. In the case of the COVID-19 pandemic, the predominant symptoms were, in descending order: fever, cough, sore throat, headache, myalgia, and arthralgia.
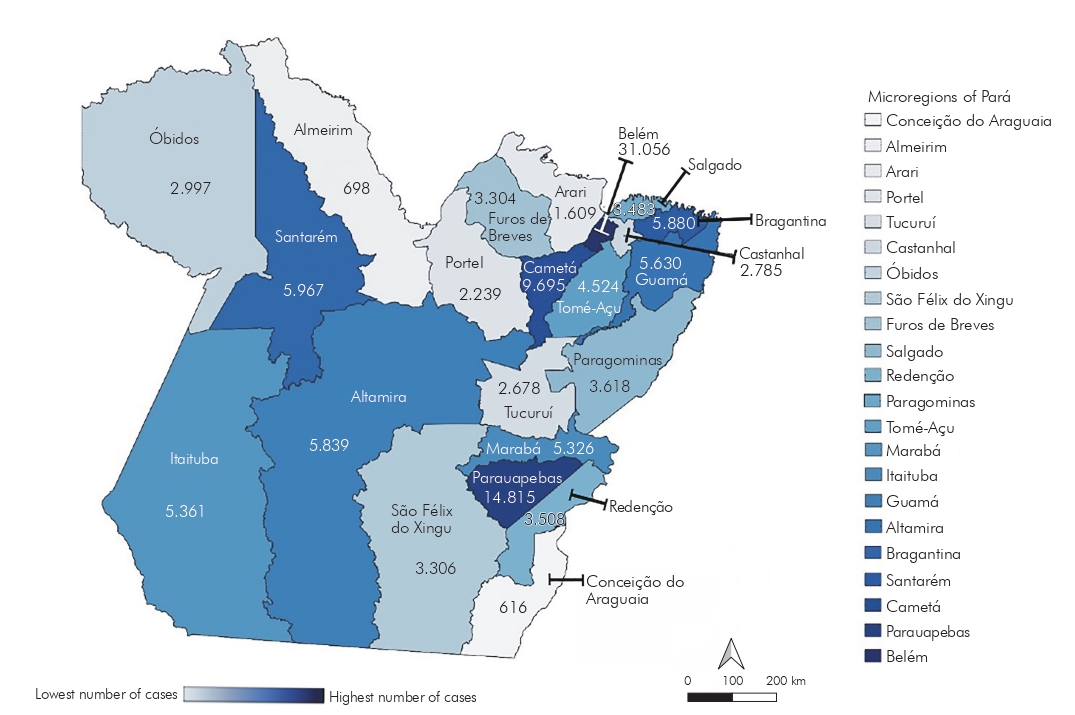
As for the spatial distribution of the respective pandemics by the microregions of the territory of Pará, a more homogeneous profile of involvement of COVID-19 was evidenced, with a greater distribution of cases in the state, compared to influenza A(H1N1)pdm09, affecting several microregions, such as the located on the outskirts of the city of Belém, capital of Pará, Guamá (5,630), and Cametá (9,695); several centers in the southwest of Pará, such as Itaituba (5,361) and Altamira (5,839); and the Baixo Amazonas, reaching the microregions Parauapebas (14,815), Óbidos (2,997), and Santarém (5,967).
Both pandemics had a high number of cases in the northeast of Pará, especially in the Belém microregion, with 86.72% (679) of the total cases for influenza A(H1N1)pdm09 and 24.86% (31,056) for COVID-19, while the microregions Conceição do Araguaia (influenza A(H1N1)pdm09 = 2; COVID-19 = 616) and Almeirim (influenza A(H1N1)pdm09 = 0; COVID-19 = 698) remained significantly less affected in both pandemics, as visualized in figures 3 and 4, which show the microregions according to the degree of incidence of cases.

Source: SINAN-DATASUS, July/2020.
Figure 3 - Incidence of influenza A(H1N1)pdm09 cases in Pará State, Brazil, from June 2009 to May 2010
DISCUSSION
The SARS-CoV-2 virus was officially detected in Brazilian territory on February 26, 2020, when the first case was notified, an older man living in the city of São Paulo, São Paulo State15. The first reported records of the Influenza A(H1N1)pdm09 virus in Brazil date back to May 7, 2009, with six confirmed cases16. Assessing the transmissibility indices (R0) for a better comparison between the pathologies, the SARS-CoV-2 initial estimates ranged from 1.6 to 4.1, reflecting a mortality rate of about 3.9/100,000 inhabitants in the country, while the Influenza A(H1N1)pdm09 virus had R0 between 1.3 and 1.8, representing mortality around 0.85/100,000 inhabitants17,18,19,20,21,22.
From the analysis of data collected in Pará, it was possible to observe a substantial difference in the impact caused between the two pandemics that occurred in recent years, even though their means of contamination and transmissibility are similar. The number of confirmed cases of COVID-19 in Pará, from March to July 2020, exceeded by more than twice the total number of cases of influenza A(H1N1)pdm09 from June 2009 to May 2010, in all the Brazilian territory, becoming the 5th State more affected by the COVID-19 pandemic, but also significantly affected by the 2009 pandemic, in which it also ranked in a prominent position, as the 6th in the number of cases. The purpose of comparing the time series carried out in this study is related to the possibility of predicting scenarios of the distribution profile of SARS-CoV-2 based on epidemiological experiences with similar characteristics due to their mechanism of contamination, infectivity, and dissemination, as in the case of the pandemic caused by the Influenza A(H1N1)pdm09 virus. Therefore, the analysis compares the differences and similarities departing from the profile of population involvement and territorial distribution of cases in Pará State among the index patients. This is done taking into account the same starting point (Figures 1 and 2) in the analysis of the infection and mortality curves, which are fundamental for the understanding and discussion in the literature regarding the multifactorial analysis regarding the impact on health services caused in each period analyzed, in addition to enabling the construction of scientific bases aimed at targeting of health surveillance measures and epidemiological monitoring in the region. The absence of an anti-SARS-CoV-2 vaccine until December 2020 and, therefore, of a mass vaccination campaign, as has occurred for years for H1N1, may justify the high numbers of cases of the new coronavirus in the population in question, as a result of the absence of pre-existing herd immunity. This immunological aspect certainly impacted the numbers of these infections by SARS-CoV-2 and Influenza A(H1N1)pdm09.
The discrepancy evidenced in raw values between the pathologies, highlighting the greater diffusion of COVID-19 in the State, can be elucidated by marked differences regarding its transmissibility. According to the report by the Centers for Disease Control and Prevention, 81% of COVID-19 cases had mild symptoms, and 1.2% were asymptomatic. Those factors facilitated the dissemination of SARS-CoV-2 due to the greater difficulty of screening the virus, unlike pathologies that have high hospitalization rates, such as MERS, and which, consequently, limit the movement of the pathogen to health facilities23. Another hypothesis favorable to its high spread is related to its different viral tropism for the respiratory tract, resulting in milder disease but highly transmissible when the virus replicates in the upper respiratory tract. When viral tropism is greater for the lower respiratory tract, there is a greater severity but less potential for propagation. This hypothesis derives from the example of the Influenza A(H1N1)pdm09 viruses, such as seasonal influenza caused by the Influenza A (H1N1) and Influenza (H3N2) viruses24,25.
In relation to other national findings on COVID-19, the data for Pará State are superior, for example, to the epidemiological survey conducted in Maranhão State26, from March to April 2020, which indicated 2,105 confirmed cases of the disease, a number about 12 times lower than in Pará, in the same time window, demonstrating that, despite the territorial proximity, each federation unit is affected in different ways when comparing the number of cases of the disease. When analyzing the data related to the influenza A(H1N1)pdm09 pandemic, the results are inferior to the findings of the observational study with 4,740 confirmed cases in Paraná State27, showing that, like COVID-19, the Influenza A(H1N1)pdm09 virus pandemic affected Brazilian regions in different ways. The variability of the incidence of influenza A(H1N1)pdm09 pandemic can be explained by the complex degree of immunological heterogeneity of local populations to circulating influenza strains, with geographic and social conditions, the degree of viral infectivity, and seasonality as transmission factors.
From the temporal analysis of incidence between pandemics, peaks of contamination and similar deaths were evidenced three months after the first notified case of the two pathologies. In the case of influenza A(H1N1)pdm09, the return of an even more ascending curve between the seventh and ninth month of the pandemic, a period that is still unknown about the COVID-19 pandemic, at the time of data collection. Among the factors listed for a new wave of infection by the Influenza A(H1N1)pdm09 virus is the seasonal characteristic of the virus28. Given the population's naive immunity to SARS-CoV-2 and its contagion potential higher than seasonal flu, the hypothesis of the emergence of a second wave of cases, in a scenario of the inexistence of effective treatments for the disease or unavailability of vaccines has been increasingly raised in recent studies29.
Comparing the incidence of the pandemics over the age group, in the case of COVID-19, the data showed a significant difference concerning the findings of Influenza A(H1N1)pdm09 virus infection, with individuals aged 30 to 39 years being the most affected by SARS-CoV-2. These results are similar to other findings, such as the epidemiological study with the population of Teresina, Piauí State, which showed that the above-mentioned age group was also the most affected, representing 32.06% of confirmed cases in that State30. A reasonable hypothesis for the prevalence in this group would be the fact that this age group is economically active, with greater exposure outside the home environment, even though pioneer studies are establishing possible degrees of cross-protection for people who came into contact with other coronaviruses in addition to the association between the severity of the disease and the blood type31,32.
Regarding the incidence of infection by the Influenza A(H1N1)pdm09 virus, there was a greater predominance of confirmed cases between 0 and 19 years of age, with 31.71% of all notified cases. This finding corroborates the findings of a cross-sectional study32 and an epidemiological survey14, in which the same age group was the most affected by influenza A(H1N1)pdm09. A possible explanation for this phenomenon is the fact that, in the 2009 pandemic, the elderly were relatively more protected, due to an immunological memory of the viral variant epitopes after being exposed to a similar strain early in life, as the one responsible for the 1918 pandemic and its variants in later years33. Therefore, such exposure over the years may have provided different degrees of immunity for individuals aged 45 years or over, thus limiting the onset at older ages and avoiding more severe symptoms in these patients34,35.
Although COVID-19 had a higher incidence among adults in Pará, the number of cases did not differ in relation to the patient's gender since there was an equivalence between the number of men (48.45%) and women (51.55%) infected. However, when the focus of the analysis was the death rate, there was a higher fatality rate among men (63.80%), with the most affected age group being 60 years old or more. Despite being relatively inferior to that described in the study by Orellana et al.36, carried out in Manaus, Amazonas State, with men over 60 years of age representing 69.1% of the total deaths, this finding is very close to those described in the international literature37,38. Although not intrinsically associated with such an outcome in this portion, the factors related to this reality are divided into biological - such as immunological differences - and behavioral, as the higher rate of smoking or neglect during quarantine39.
As for the influenza A(H1N1)pdm09 pandemic, there was a higher incidence of cases (60.92%) and deaths (75.61%) in females. Such data confront the relative equivalence of the involvement of women by the virus in other studies40,41, possibly related to inconsistencies in official data or even to underreporting of cases among men, since, historically, this group tends to seek less the health units for care42.
It was possible to observe a similarity in the symptoms between the two pathologies, emphasizing the presence of fever associated with respiratory problems such as cough. In 2011, Khandaker et al.43 corroborated this fact by highlighting, in their systematic review, that 84% of confirmed cases had cough and fever as the most prevalent symptoms in those infected with the Influenza A(H1N1)pdm09 virus, being classified as important markers for screening for similar respiratory diseases. Furthermore, the similarity of the clinical findings extends, according to the data collected, to symptoms such as myalgia and arthralgia, reported in a significant number of cases of COVID-19 in the State, a factor also reported in another study44, which added fatigue, gastrointestinal symptoms, and productive cough as preponderant factors for patients with SARS-CoV-2, a condition also registered in people infected with the Influenza A(H1N1)pdm09 virus.
Regarding the evolution of symptoms and the severity related to the need for hospitalization, there was a high rate of cases of SARS with hospitalization (79.43%) caused by the Influenza A(H1N1)pdm09 virus in 2009, almost double the finding in a study by Lenzi et al.45, carried out with 4,740 patients with laboratory confirmation, in Paraná State, of which 40.3% were hospitalized. Among the factors associated with the fact, it is noteworthy that, as of 2009, only cases of hospitalizations due to SARS were reported in the SINAN database (SINAN Influenza Web)46.
Among the factors associated with the control of the number of new cases and deaths by COVID-19 are the interruption of non-essential activities during the period of lockdown (from 07/05/2020 to 24/05/2020) and the adoption of the use of masks as a mandatory measure, under penalty of fine47,48. That was because of the publication of new studies associating high exposures to the SARS-CoV-2 inoculum with greater penetration into the lower respiratory tract, which would be related to the worsening of the disease, while lower exposures to the inoculums, due to the physical barrier of the masks, allow contact with a lower viral load, increasing the time for the immune response and resulting in a milder infection49,50.
From comparing the spatial incidence between the two pandemics, it was possible to observe the percentage concentration of cases in the Belém microregion, having, respectively, 86.72% and 24.86% of the total notifications of cases of influenza A(H1N1)pdm09 and COVID-19. Among the factors associated with greater homogeneity in the incidence of COVID-19 cases among the microregions of the State is the issue of the long incubation period for SARS-CoV-2 (from one to 14 days), compared to the Influenza A(H1N1)pdm09 virus (from one to seven days), favoring contagion, as asymptomatic people are potential sources of contamination51,52. Another point to be considered concerns the substantial difference in the number of tests performed in the two pandemics. In the COVID-19 pandemic, this number exceeded approximately 60 times the testing carried out in 2009, and, therefore, the greatest potential for screening is associated with the lowest underreporting of cases. In addition, it is important to emphasize, as a factor for inducing immunity and lower circulation of pandemic viruses, that the Influenza A(H1N1)pdm09 virus has seasonally circulating strains and as part of the annual vaccine, strains that suffer frequent mutations in their RNA (drifts)53.
It is important to note that Pará has microregions with significant population rates, allowing greater dissemination of SARS-CoV-2, according to studies carried out by the WHO54. A possible hypothesis for internalization, according to Ribeiro et al.55, is due to the connection between Brazilian cities of different sizes and complexities through the air, road, and river networks, which allows the contact of people from the most diverse locations, a fact that occurs in the metropolitan region of Pará, especially in the microregion where is the capital, one of the main economic centers of the Amazon Region, favoring the interiorization of COVID-19.
Given the use of secondary databases, this study recognizes the limitations related to the detailing of notified cases, reiterating, in addition to its focus on the participation of complementing the epidemiological panoramas already developed regarding the aforementioned pandemics, the need for constant updating of research focused on the theme, in view of the seriousness related to the current epidemiological scenario.
CONCLUSION
The results found in this study demonstrate the discrepancy in the impact of both pathologies in Pará State, with COVID-19 showing a number of infected people approximately 60 times higher than influenza A(H1N1)pdm09. The superiority in the values found is also reflected in other factors such as the mortality rate and the spatial distribution in the State. It is worth highlighting the differences in incidence and fatality according to age group, with the most prevalent cases of Influenza A(H1N1)pdm09 virus in children and young people between 10 and 19 years old and higher mortality between 20 and 29 years old, while for the SARS-CoV-2 was observed in adults, between 30 and 39 years old, with significant mortality in the age group above 60 years. The epidemiological comparison of the time series in the present study allows us to foresee different scenarios of the impact of each pathology, such as the territorial distribution and dissemination of each pathology, based on the analysis of its first paired cases. Thus, these findings highlight that the documented experience of the influenza A(H1N1)pdm09 pandemic in Pará reflects the need to change the strategic planning that should be implemented in the COVID-19 pandemic, in view of affecting different publics and their pathophysiological differences, it enhances the need for greater support from public policies to improve the capacity to provide medical care to the population and epidemiological surveillance in the Amazon Region.
REFERENCES
1 Saunders-Hastings PR, Krewski D. Reviewing the history of pandemic influenza: understanding patterns of emergence and transmission. Pathogens. 2016 Dec;5(4):66. [ Links ]
2 Guedes ALS, Santos DLS, Fernandes Jr EL, Sousa GT, Vieira NP, Oliveira IP. Análise epidemiológica da situação da influenza pandêmica (h1n1) 2009/2010 no estado de Goiás. Rev Eletronica Fac Montes Belos. 2011;4(2). [ Links ]
3 Cruz GMA, Lima RC, Costa DO, Bastianini LFM. H1N1 vírus: perfil epidemiológico do vírus no período da pandemia de 2009 e 2010 nas cinco regiões brasileiras. Rev Eletronica FACIMEDIT. 2016 dez-2017 jan;6(2):53-66. [ Links ]
4 Lofgren E, Fefferman NH, Naumov YN, Gorski J, Naumova EN. Influenza seasonality: underlying causes and modeling theories. J Virol. 2007 Jun;81(11):5429-36. [ Links ]
5 Valleron A-J, Cori A, Valtat S, Meurisse S, Carrat F, Boëlle P-Y. Transmissibility and geographic spread of the 1889 influenza pandemic. Proc Natl Acad Sci USA. 2010 May;107(19):8778-81. [ Links ]
6 Kind L, Cordeiro R. Narrativas sobre a morte: a gripe espanhola e a Covid-19 no Brasil. Psicol Soc. 2020;32:e020004. [ Links ]
7 Thacker SB. The diffusion of influenza: patterns and paradigms. JAMA. 1987;258(3):389. [ Links ]
8 Cockburn WC, Delon PJ, Ferreira W. Origin and progress of the 1968-69 Hong Kong influenza epidemic. Bull World Health Organ. 1969;41(3):345-8. [ Links ]
9 Silva HRC, Kazikawa GT, Pinheiro JPS, Santos FAL, Isoton DA. Análise epidemiológica da pandemia pelo Influenza A (H1N1) no Brasil nos anos de 2009 a 2010 [trabalho de conclusão de curso]. Várzea Grande (MT): UNIVAG; 2018. [ Links ]
10 Costa LMC, Merchan-Hamann E. Pandemias de influenza e a estrutura sanitária brasileira: breve histórico e caracterização dos cenários. Rev Pan-Amaz Saude. 2016 mar;7(1):11-25. [ Links ]
11 Cugini DM, Silva FPA, Éttori H, Krumenauer MZ, Moreira ME, Paulucci RS. Perfil epidemiológico dos casos de influenza A H1N1 em Taubaté-SP. Bol Epidemiol Paul. 2010 set;7(81):17-25. [ Links ]
12 Rossetto ÉV, Luna EJA. Clinical aspects of influenza A(H1N1) pdm09 cases reported during the pandemic in Brazil, 2009-2010. Einstein (Sao Paulo). 2015 Apr-Jun;13(2):177-82. [ Links ]
13 Senhoras EM. Coronavírus e o papel das pandemias na história humana. Bol Conjunt. 2020;1(1):31-4. [ Links ]
14 Schuelter-Trevisol F, Dutra MC, Uliano EJM, Zandomênico J, Trevisol DJ. Perfil epidemiológico dos casos de gripe A na região sul de Santa Catarina, Brasil, na epidemia de 2009. Rev Panam Salud Publica. 2012 jul;32(1):82-6. [ Links ]
15 Ministério da Saúde (BR). Ministério da Saúde declara transmissão comunitária nacional [Internet]. Brasília: Ministério da Saúde; 2020 [citado 2020 abr 7]. Disponível em: Disponível em: https://www.gov.br/saude/pt-br/assuntos/noticias/ministerio-da-saude-declara-transmissao-comunitaria-nacional . [ Links ]
16 Machado AA. Infecção pelo vírus Influenza A (H1N1) de origem suína: como reconhecer, diagnosticar e prevenir. J Bras Pneumol. 2009 mai;35(5):464-9. [ Links ]
17 Read JM, Bridgen JRE, Cummings DAT, Ho A, Jewell CP. Novel coronavirus 2019-nCoV: early estimation of epidemiological parameters and epidemic predictions. MedRxiv. 2020 Jan 28. [ Links ]
18 Liu T, Hu J, Kang M, Lin L, Zhong H, Xiao J, et al. Transmission dynamics of 2019 novel coronavirus (2019-nCoV). BioRxiv. 2020 Jan 26. [ Links ]
19 Cao Z, Zhang Q, Lu X, Pfeiffer D, Jia Z, Song H, et al. Estimating the effective reproduction number of the 2019-nCoV in China. MedRxiv . 2020 Jan 29. [ Links ]
20 Our World in Data. COVID-19 data explorer: daily new confirmed COVID-19 cases per million people [Internet]. 2020 [cited 2021 Mar 5]. Available from: Available from: https://ourworldindata.org/coronavirus-data-explorer . [ Links ]
21 Secretaria de Estado da Saúde (São Paulo). Coordenadoria de Controle de Doenças. Influenza A/H1N1 novo subtipo viral. Bol Epidemiol Paul. 2009 jun;6(66):17-23. [ Links ]
22 Fundação Oswaldo Cruz. Monitoramento de casos de síndrome respiratória aguda grave (SRAG) notificados no SIVEP-Gripe [Internet]. 2019 [citado 2021 fev 3]. Disponível em: Disponível em: http://info.gripe.fiocruz.br/ . [ Links ]
23 Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020 Mar;38(1):1-9. [ Links ]
24 Chen J. Pathogenicity and transmissibility of 2019-nCoV-A quick overview and comparison with other emerging viruses. Microbes Infect. 2020 Mar;22(2):69-71. [ Links ]
25 Petrosillo N, Viceconte G, Ergonul O, Ippolito G, Petersen E. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect. 2020 Jun;26(6):729-34. [ Links ]
26 Almeida JS, Cardoso JA, Cordeiro EC, Lemos M, Araújo TME, Sardinha AHL. Caracterização epidemiológica dos casos de Covid-19 no Maranhão: uma breve análise. SciELO Preprints. 2020 mai 5. [ Links ]
27 Lenzi L, Mello AM, Silva LR, Grochocki MHC, Pontarolo R. Influenza pandêmica A (H1N1) 2009: fatores de risco para o internamento. J Bras Pneumol. 2012 jan-fev;38(1):57-65. [ Links ]
28 Biggerstaff M, Cauchemez S, Reed C, Gambhir M, Finelli L. Estimates of the reproduction number for seasonal, pandemic, and zoonotic influenza: a systematic review of the literature. BMC Infect Dis. 2014 Sep;14:480. [ Links ]
29 Wang Q, Zhang T, Zhu H, Wang Y, Liu X, Bai G, et al. Characteristics of and public health emergency responses to COVID-19 and H1N1 outbreaks: a case-comparison study. Int J Environ Res Public Health. 2020 Jun;17(12):4409. [ Links ]
30 Araújo AAC, Amaral JV, Sousa JN, Fonseca MCS, Viana CMC, Mendes PHM, et al. Covid-19: análise de casos confirmados em Teresina, Piauí, Brasil. Rev Prev Infec Saude. 2020;6:10569. [ Links ]
31 Jhaveri R. Echoes of 2009 H1N1 influenza pandemic in the COVID pandemic. Clin Ther. 2020 May;42(5):736-40. [ Links ]
32 Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, Invernizzi P, et al. Genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med. 2020 Oct;383(16):1522-34. [ Links ]
33 Fajardo-Dolci G, Gutierrez-Vega R, Arboleya-Casanova H, Villalobos A, Wilson KS, García SG, et al. Clinical characteristics of fatalities due to influenza A (H1N1) virus in Mexico. Thorax. 2010 Jun;65(6):505-9. [ Links ]
34 Xu R, Ekiert DC, Krause JC, Hai R, Crowe Jr JE, Wilson IA. Structural basis of preexisting immunity to the 2009 H1N1 pandemic Influenza virus. Science. 2010 Apr;328(5976):357-60. [ Links ]
35 Miller MA, Viboud C, Balinska M, Simonsen L. The signature features of influenza pandemics - implications for policy. Engl J Med. 2009 Jun;360(25):2595-8. [ Links ]
36 Orellana JDY, Cunha GM, Marrero L, Horta BL, Leite IC. Explosion in mortality in the Amazonian epicenter of the COVID-19 epidemic 19. Cad Saude Publica. 2020;36(7):e00120020. [ Links ]
37 Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 May;323(18):1775-6. [ Links ]
38 Ghislandi S, Muttarak R, Sauerberg M, Scotti B. News from the front: estimation of excess mortality and life expectancy in the major epicenters of the COVID-19 pandemic in Italy. MedRxiv. 2020 Jun 20. [ Links ]
39 Lima DLF, Dias AA, Rabelo RS, Cruz ID, Costa SC, Nigri FMN, et al. Covid-19 in the state of Ceará: behaviors and beliefs in the arrival of the pandemic. Cien Saude Colet. 2020 May;25(5): 1575-86. [ Links ]
40 Oliveira WK, Carmo EH, Penna GO, Kuchenbecker RS, Santos HB, Araújo WN, et al. Pandemic H1N1 influenza in Brazil: analysis of the first 34,506 notified cases of influenza-like illness with severe acute respiratory infection (SARI). Euro Surveill. 2009 Oct;14(42):19362. [ Links ]
41 Punpanich W, Chotpitayasunondh T. A review on the clinical spectrum and natural history of human influenza. Int J Infect Dis. 2012 Oct;16(10):e714-23. [ Links ]
42 Romeu G, Nascimento EF, Araújo FC. Por que os homens buscam menos os serviços de saúde do que as mulheres? As explicações de homens com baixa escolaridade e homens com ensino superior. Cad Saude Publica. 2007 mar;23(3): 565-74. [ Links ]
43 Khandaker G, Dierig A, Rashid H, King C, Heron L, Booy R. Systematic review of clinical and epidemiological features of the pandemic influenza A (H1N1) 2009. Influenza Other Respir Viruses. 2011 May;5(3):148-56. [ Links ]
44 Tang X, Du R-H, Wang R, Cao T-Z, Guan L-L, Yang C-Q, et al. Comparison of hospitalized patients with ARDS caused by COVID-19 and H1N1. Chest. 2020 Jul;158(1):195-205. [ Links ]
45 Lenzi L, Mello AM, Silva LR, Grochocki MHC, Pontarolo R. Influenza pandêmica A (H1N1) 2009: fatores de risco para o internamento. J Bras Pneumol. jan-fev 2012;38(1):57-65. [ Links ]
46 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Situação epidemiológica da Influenza Pandêmica (H1N1) 2009 no Mundo e no Brasil, até a Semana Epidemiológica 47 de 2009. Informe Epidemiol Influenza Pandemica (H1N1). 2009 dez;1(11):1-11. [ Links ]
47 G1 PA. Belém e mais 9 cidades do Pará entram em "lockdown"; estado é o 2º do país a adotar a medida contra o coronavírus. G1 [Internet]. 2020 mai 7 [citado 2021 jul 13]. Disponível em: Disponível em: https://g1.globo.com/pa/para/noticia/2020/05/07/belem-e-mais-9-cidades-do-para-entram-em-lockdown-estado-e-o-2o-do-pais-a-adotar-a-medida-contra-o-coronavirus.ghtml . [ Links ]
48 G1 PA. Lockdown em Belém e mais nove municípios do Pará é prorrogado até 24 de maio. G1 [Internet]. 2020 mai 15 [citado 2021 jul 13]. Disponível em: Disponível em: https://g1.globo.com/pa/para/noticia/2020/05/15/lockdown-no-para-e-prorrogado-ate-24-de-maio.ghtml . [ Links ]
49 Bielecki M, Züst R, Siegrist D, Meyerhofer D, Crameri GAG, Stanga Z, et al. Social distancing alters the clinical course of COVID-19 in young adults: a comparative cohort study. Clin Infect Dis. 2020 Feb;72(4):598-603. [ Links ]
50 Gandhi M, Beyrer C, Goosby E. Masks do more than protect others during COVID-19: reducing the inoculum of SARS-CoV-2 to protect the wearer. J Gen Intern Med. 2020 Oct;35(10):3063-6. [ Links ]
51 Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Doenças infecciosas e parasitárias: guia de bolso. 8. ed. rev. Brasília: Ministério da Saúde; 2010. 442 p. [ Links ]
52 Samudrala PK, Kumar P, Choudhary K, Thakur N, Wadekar GS, Dayaramani R, et al. Virology, pathogenesis, diagnosis and in-line treatment of COVID-19. Eur J Pharmacol. 2020 Sep;883:173375. [ Links ]
53 Forleo-Neto E, Halker E, Santos VJ, Paiva TM, Toniolo-Neto J. Influenza. Rev Soc Bras Med Trop. 2003 mar-abr;36(2):267-74. [ Links ]
54 World Health Organization. Coronavirus disease 2019 (COVID-19): situation report -38 [Internet]. Geneva: World Health Organization; 2020 [cited 2021 Mar 9]. Available from: Available from: https://apps.who.int/iris/handle/10665/331226 . [ Links ]
55 Ribeiro SP, Silva AC, Dáttilo W, Reis AB, Góes-Neto A, Alcantara LCJ, et al. Severe airport sanitarian control could slow down the spreading of COVID-19 pandemics in Brazil. PeerJ. 2020 Jun;8:e9446. [ Links ]
How to cite this article / Como citar este artigo: Geha YF, Coutinho FM, Marvão MCR, Nogueira TLP, Mota ACC, Lucena CCC, et al. Comparative epidemiological analysis between pandemics caused by the Influenza A(H1N1)pdm09 and SARS-CoV-2 viruses in Pará State, Brazil. Rev Pan Amaz Saude. 2021;12:e202100862. Doi: http://dx.doi.org/10.5123/S2176-6223202100862
Received: December 22, 2020; Accepted: May 28, 2021











 text in
text in