INTRODUCTION
Rotaviruses (RVs) are major diarrheal pathogens that make up a large, genetically diverse genus of double-stranded RNA viruses belonging to the Reoviridae family. They are classified into groups, subgroups, and serotypes/genotypes1,2. According to their antigenic properties, rotavirus (RV) strains can be classified into groups of A-J and subgroups (SG) I, II, I + II, non-I e non-II3,4. SG II is more prevalent among humans, while SG I is more frequently detected in animals5,6. Currently, group A RVs comprise at least 36 G types and 52 P types known to infect humans and animals. G3 types are commonly found in humans and different animals like cats, dogs, monkeys, pigs, rabbits, and horses7.
RVs depict an icosahedral symmetry, are non-enveloped viruses, and have inner and outer capsid layers, from which 32 capsomers radiate surrounding 11 segments of ds RNA (16-21 kbp)8,9,10. Genetically, RVs are diverse with several genotypes typically distributed across humans and various animal species, suggesting that host species barriers exist, as well as host range restrictions according to the reservoir species. In addition, feline RVs with epidemiological importance in zoonotic infections have been reported and have been classified into at least two different groups, comprising the feline/human AU-1/FRV1-like G3P[9] viruses (appear to form a highly homogeneous group) and the canine/feline/human G3P[3] viruses (appear to be genetically heterogeneous)11. Of note, there have been reports of RV strains isolated from humans and animals, including birds and mammals, that share genetic and antigenic properties across heterologous species12,13,14,15.
Clinically, gastrointestinal illnesses caused by group A RVs and other viruses, such as coronavirus, are reported in domestic cats and dogs, which in general evolve with nonspecific symptoms, including vomiting and dehydration from acute watery diarrhea12,13,16,17.
RVs are important gastroenteritis agents in children under 5 years of age, as well as the cause of neonatal diarrhea associated with significant morbidity and mortality worldwide. In humans, deaths occur less frequently in developed countries. Furthermore, in several wild and domestic animal species, RV infections are associated with delayed growth, increased susceptibility to other diseases, and increased mortality2,10,18. In addition, RVs have been found as a cause of gastroenteritis in domestic cats in several countries, since the 1970s19,20,21.
This case study presents the first confirmation of RV infection in wild cats of the species Leopardus Tigrinus (Schreber, 1775), the smallest wild Brazilian felid and one of the least known among Neotropical felids, and Leopardus pardalis (Linnaeus, 1758) in the Amazon22. This report aims to discuss the RV disease course in these orphaned carnivores kept in captivity, while rehabilitation quarantine. This report also aims to contribute to the studies concerning the evolution and epidemiology of RV infections across animal species by recording two clinical cases of simultaneous occurrence and proposed horizontal transmission of RV.
RV infections in both domestic and wild cats may be related to the occurrence of strains homologous to strain AU228 which is also potentially infectious for humans23. In this regard, the disease among humans might be considered as emerging, possibly resulting from the adaptation of feline RVs, a process that involves both genetic and evolutionary interactions between strains, which have recently been highlighted through the advent of new diagnostic tools that allow classification based on the complete genome sequencing enables phylogenetic analyses toward the determination of RV genotypes24,25,26,27.
The existence of enteric viruses infecting animals seems to denote potential reservoirs for infections in humans and, consequently, the study of these viral pathogens in animals may be of key importance for a better understanding of the evolution of these agents, as well as in shedding light in the process of possible breaking down interspecies barrier28,29,30,31.
RV infections can evolve asymptomatically, but hosts can shed the virus in their feces, serving as a possible reservoir for viruses in their natural habitat21,32. Although the natural transmission of RV between animal species is still the subject of studies and remains controversial, the possibility should not be overlooked in the context of epidemiological studies33.
Previous studies have shown the frequency of RVs and other enteric viruses in wild animals in the Amazon Region (marsupials, rodents, chiropterans, and primates), evidencing positive samples were both from the "common opossum", Didelphis marsupialis Linnaeus, 175834. More recently, studies focused on the prevalence of G3 equine-like, G6, G1 (VP7 gene), P[2] (VP4 gene), T2 (NSP3 gene) genotypes of RVA, avian RV (A, D, F), and picobirnavirus in wild and exotic avian circulating in the Amazonian urban habitat35. Therefore, the results of epidemiologic data in the wild population cannot be dismissed as new insight into the potential influence of RVs epidemiology and the role of wild and exotic hosts as potential reservoirs of the broad range of genotypes, usually considered typical solely from domestic animals and humans35. Nevertheless, when compared to the biodiversity of the Amazon biome and given the present anthropic transformations of the region, epidemiological data on the dynamics of enteric viruses in domestic and wildlife of the Amazon Region are still scarce35,36,37,38. Thus, among other similar reports, these findings appear to be important in the evaluation of the epidemiological profile of enteric viruses associated with infections in animal populations either in situ on preserved habitat or from areas degraded by anthropic action37,38,39,40.
CASE REPORT
This research was licensed by the Biodiversity Information and Authorization System (Sistema de Autorização e Informação em Biodiversidade - SISBIO) under Nº. 39285, and by the Ethics Committee on Animal Use of Universidade Federal Rural da Amazônia (UFRA), authorization Nº. 034/2014.
The Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA) service agents rescued and sent to the Veterinary Sector of the Mangal das Garças Environmental Park (SV-PAMG), located in the Municipality of Belém (01°27'21" S and 48°30'16" W), Pará State, Amazon, Brazil, two orphaned male felines of species L. tigrinus, which is an endangered species, and L. pardalis; both species are known to display a wide geographical distribution in Central and South America39.
The L. pardalis, 3 months old, was kept illegally in a residence in the Municipality of Altamira (03°12'12" S and 52°12'23" W)39, Pará State. The animal was kept in the SV-PAMG together with an orphaned L. tigrinus of the same age, which had been received two months before by IBAMA environmental agency agents at the City of Chaves (00°09'36" S, 49°59'16" W), Marajó Island, Pará State, Brazil.
At the SV-PAMG, restrictive quarantine protocols for long-term care facilities were implemented, in considering the physical and biological needs of both species. A nutritional and behavioral assessment form was prepared for the daily recording of data during the quarantine and captivity of the cubs.
Both animals were clinically evaluated during the period of quarantine, and, after an adaptation period of two months to quarantine and management in captivity, the L. pardalis began to present episodes of postprandial emesis and rapid progression to cachexia and dehydration. A week later, watery yellowish diarrhea developed with foul-smelling stools. During this period, the L. tigrinus had also started to pass diarrheic stools.
During the symptomatic phase, the animals received daily support therapy with fluid replacement (electrolytes and vitamin complexes), symptomatic treatment including the administration of bromopride and loperamide hydrochloride, and chemotherapy treatment including enrofloxacin and metronidazole.
Twelve days after the onset of clinical signs and symptoms, the L. pardalis died, but the necropsy findings were insufficient to determine the specific cause of the infection. Concomitantly, the L. tigrinus, still undergoing intensive clinical treatment, was subjected to blood testing procedures. The samples were collected in appropriate blood collection tubes (Vacutainer®) containing ethylenediaminetetraacetic acid for hemogram, as well as stool samples (collection made in the routine flask for clinical analysis) for enteric diarrheagenic pathogens detection at the Virology Laboratory at the Instituto Evandro Chagas, Pará State.
The stool samples were stored at -20 ºC until being processed by using immunochromatography (ICG), enzyme-linked immunosorbent assay (ELISA), polyacrylamide gel electrophoresis (PAGE), and reverse transcriptase polymerase chain reaction (RT-PCR) methods, starting with the preparation of suspensions (5% and 10%, weight/volume) and tested strictly as per manufacturers' instructions. Briefly, the ICG involved the commercial kit Rota-Strip© (CORIS, BioConcept, Glemboux, Belgium), containing nitrocellulose strips sensitized with monoclonal antibodies directed to group A RV proteins. The suspension under test and a complex involving antibodies monoclonal specifics for RVs coupled with particulate gold, migrate together through passive diffusion along the ribbon, resulting in a positive reaction highlighted by the appearance of a bluish-red color line. The ELISA used the commercial kit RIDASCREEN© (R-Biopharm AG, Darmsdat, Germany), which consists of the use of microplates previously sensitized with specific monoclonal antibodies targeted at the viral protein VP6. Group A RV antigen was identified in the fecal sample of L. tigrinus, which also died 13 days after the onset of the first symptoms. The complete blood count of this animal showed normocytic and normochromic anemia (Ht 21.1%; Hm 3,970,000/mm3; Hb 7.8), with reactive leukocytosis (23,340/mm3). To molecular technique, fecal suspensions were prepared in 10% (w/v) in phosphate-buffered saline (PBS 1X pH 7.4), clarified by centrifugation at 10,500 x g for 20 min at 4 ºC followed by the extraction of nucleic acid (dsRNA) using silica glass powder as described by Boom et al.41. PAGE and silver staining were performed42. The RT-PCR to RVA was conducted using specific primers Con2/Con3 for the VP4 to amplify 876 bp43 and Beg 9/End 9 for the VP7 genes to amplify 1,062 bp44, with SuperScriptTM (Invitrogen, Carlsbad, CA) and recombinant Taq DNA polymerase (Invitrogen).
Both ICG and ELISA assays yielded group A RV antigen-positive results. Based on these results, the animal was promptly subjected to treatment with Nitazoxanide and, after 13 days of convalescence, the animal died. The RVA positive samples were subjected to PAGE and RT-PCR for VP4 and VP7 genes using conventional set primers but yielded negative.
Stool samples from the animals were examined for ova and parasites yielding negative results as to the presence of intestinal endoparasites. In addition, stool samples were tested by different conventional molecular methodologies for RVs; however, possibly owing to false-negative or to low viral loads positive results were not yielded.
Following death, both animals were necropsied at Animal Pathology Laboratory (LABOPAT) of UFRA. In the ectoscopic examination, L. pardalis showed signs of emaciation, pale mucous membranes (Figure 1A), and mucousy stools adhered to the perianal region (Figure 1B). In their small intestine, the mucosa was hyperemic and swollen, and mucus was abundant (typical of catarrhal enteritis).
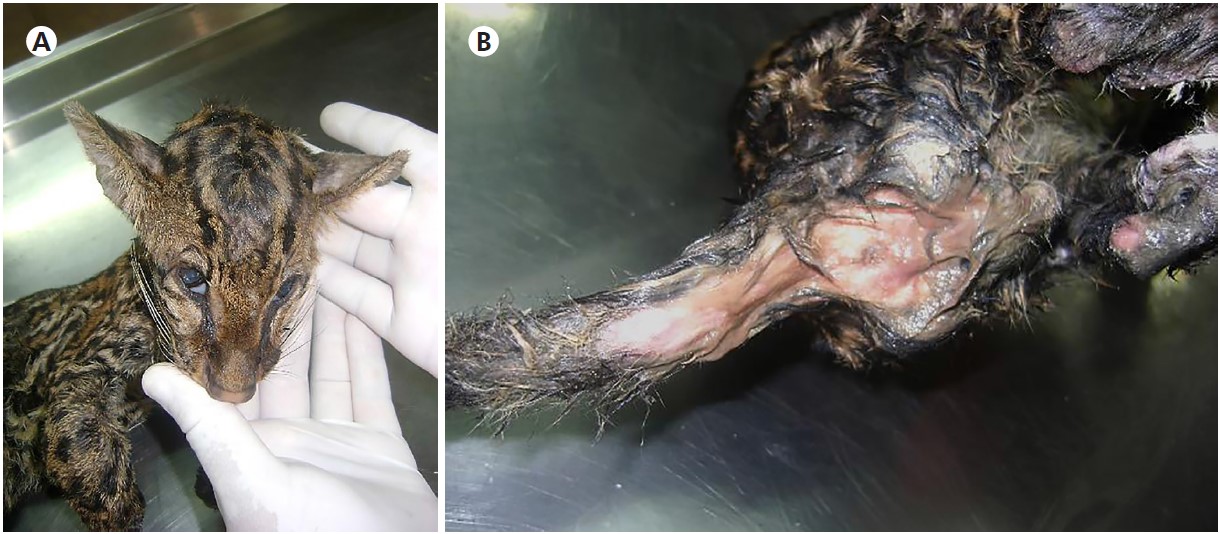
Source: Digital archive of the LABOPAT/UFRA.
A: L. pardalis depleted state, dehydrated, and pale conjunctiva; B: Very humid perianal region due to mucoid diarrhea.
Figure 1 - Ectoscopic examination of L. pardalis during necropsy
L. tigrinus also presented cachexia, conjunctival mucosa pallor (Figure 2A), in addition to citrus, slightly cloudy-yellow liquid in the abdominal cavity (Figure 2B), containing small fibrinous lumps (a characteristic feature of serofibrinous peritonitis). The cytology of this liquid confirmed peritonitis due to the prominent presence of neutrophils and an increase in macrophage counts (Figure 2C). In the small intestine, changes were predominant in the jejunum, with a notable presence of mucus mixed with haemolysed blood, which are findings characteristic of hemorrhagic catarrhal enteritis (Figure 2D).
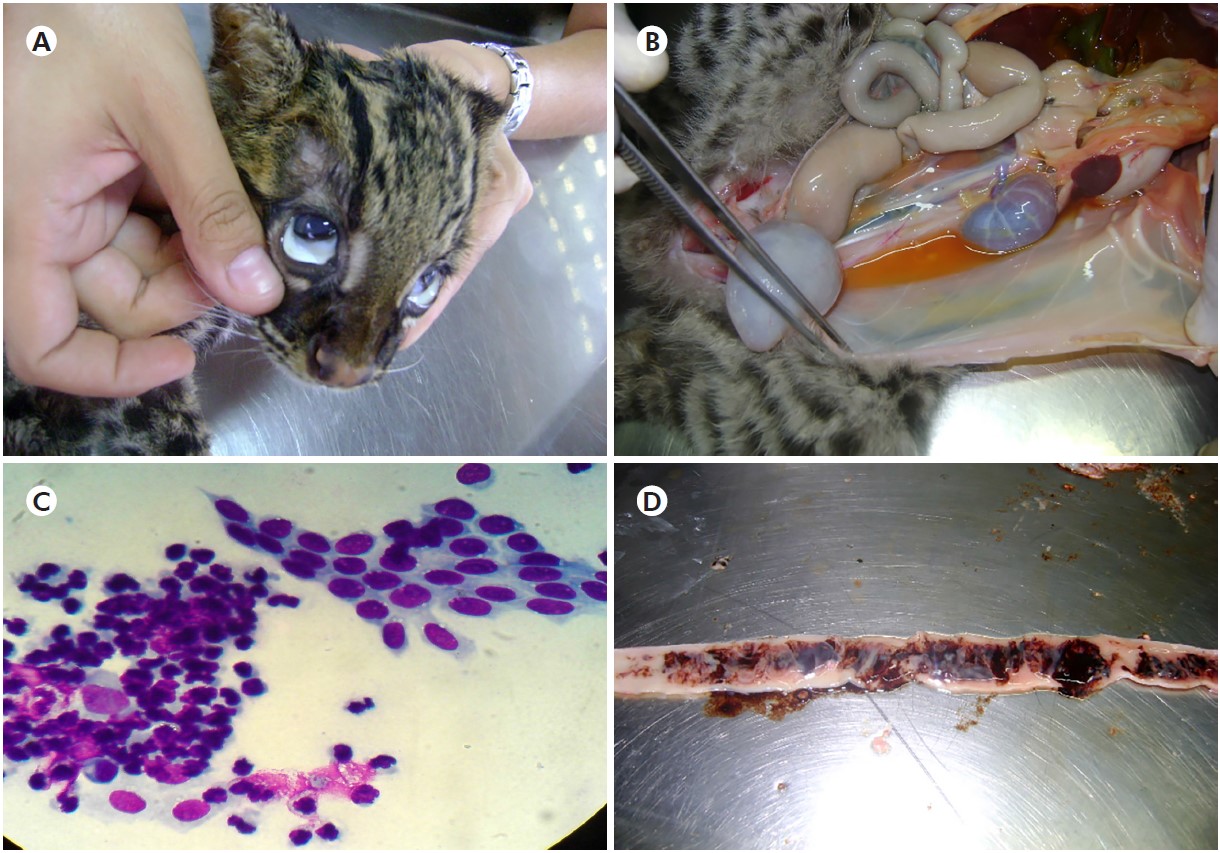
Source: Digital archive of the LABOPAT/UFRA.
A: L. tigrinus presenting conjunctival mucosa pallor; B: Increased citrus yellow fluid into the abdominal cavity; C: Peritoneal cytology, showing mesothelial cells and increased neutrophils; D: Duodenum with mucus-hemorrhagic exudate.
Figure 2 - Ectoscopic examination of L. tigrinus during necropsy and cytological findings
The duodenal villi of both animals showed an increase in desquamation and typically more dilated intestinal crypts; this strongly suggests an acute RV infection. In the ileum of L. tigrinus, the villi were shorter with noticeable desquamation, and the dilated crypts showed necrotic remains (Figure 3A), together with erythrocytes, concurrently with the regeneration stage of villus epithelial cells and infiltration of lymphocytes in inter villous areas (Figure 3B). Histologically, no major changes were observed in the other organs.
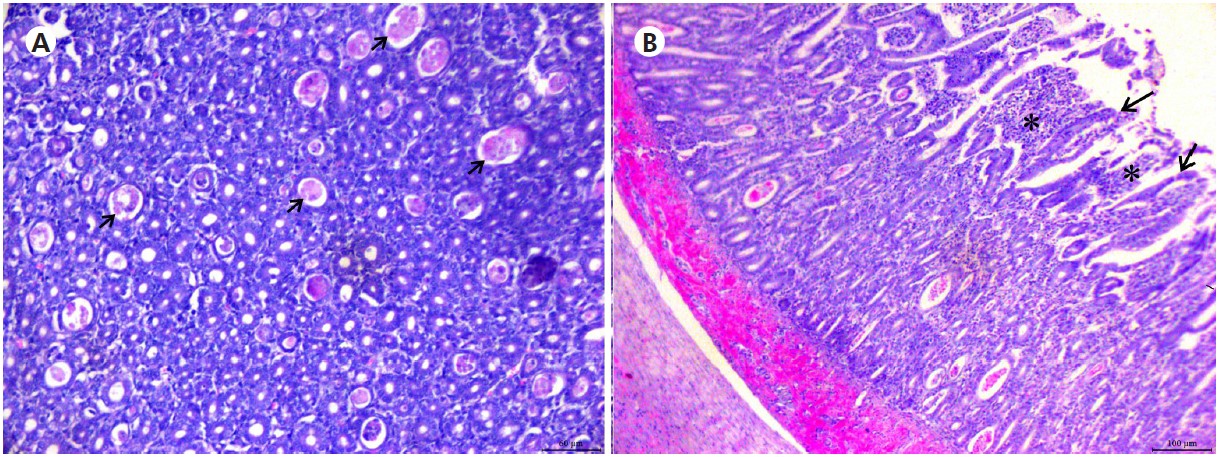
Source: Digital archive of the LABOPAT/UFRA.
A: Photomicrograph of the ileum with dilated crypts showed necrotic remains (arrows). Bar = 60 μm; B: Section from the duodenum with shortened villi (arrows) and infiltration of lymphocytes in intervillous areas (*). Bar = 100 μm.
Figure 3 - Hematoxylin-eosin stained thin L. tigrinus intestinal sections
DISCUSSION
L. tigrinus, a species considered at risk of extinction by the International Union for Conservation of Nature, and L. pardalis are Neotropical felids that suffer constant anthropic pressure mainly due to habitat loss and fragmentation caused by agricultural expansion45. Cubs of these and other wild mammals are frequently rescued by the environmental inspection bodies in the Amazon and destined for care in Brazilian fauna conservation institutions39,40.
In this context, it has been reported that the direct contact of these animals with humans and other species, including domestic, can facilitate the spread of emerging diseases, affecting new hosts and notifications for new geographic occurrences, as reported from infections by parasites, coronavirus, retrovirus, feline leukemia virus, and feline immunodeficiency virus in wild cats, among other pathogens46,47,48,49. Reinforcing the need for more studies to be developed in this area, based mainly on the complete genetic sequencing of the pathogens, which can raise discussions about the occurrence (or not) of interspecies heterologous transmission1,10,50,51,52.
Evidence of interspecies transmission and genetic reassortments between human and animal RVs have been reported elsewhere38. Some animal species seem to contribute frequently to this antigenic/genetic broad diversity found in the context of human RV infections, presumably because of close interactions with other animal species, including domestic ones1,38.
Group A RVs are the most important epidemiologically because they are associated with a higher number of infections in humans and a broad range of mammals and birds, including interspecies transmission53. Historically, groups A, B, and C RVs are known to infect humans and other mammals37,53, whereas groups D, F, and G RVs were found only in birds54,55. Nevertheless, there are reports of avian group A RVs, which generally fall into a separate genotype and show different electrophoretic patterns from RVs that infect mammals, suggesting that transmission of these avian viruses to mammals would be unlikely55,56. In view of this, it seems plausible to assume that no relationship exists in terms of transmission from birds of the PAMG to the two quarantined wild cats. However, it is worth mentioning that group A RVs of avian origin have already been found in other bird species such as chickens, ducks, pigeons, ostriches, pheasants, and other wild birds55,56. In Brazil, these RVs were first identified in broiler chicken fecal samples and, since then, few studies have been developed in Amazonia aiming at the detection of these agents in birds35.
In mammals, it is worth mentioning the first detection in the Amazon of RVs which displayed a typical group A RNA electropherotype, in wild marsupials34. There have been detection of around 10% infections in pigs caused by group A RVs in the Metropolitan Region of Belém, with the predominance of G3 and G5 genotypes, also bearing P genotypes P[13]/[22] and P[23]57. Two of these RVA strains were characterized to have VP3 genes of human origin possessing DS-1-like backbone, suggesting to have evidence for human-to-porcine zooanthroponotic transmission58. In contrast, in developing countries, including Asian and sub-Saharan African countries, RV strains of human origin displaying unusual G and P combinations have been seen frequently, including reassortment events involving RVs strains of animal origin38,59.
Although pigs and cattle may harbor group A RV infection sub-clinically at low frequency, further studies are urgently needed to better understand the dynamic of zoonotic risk involving transmission to humans60,61. Of note, the porcine genogroup E RVs are considered uncommon and its pathogenesis is not yet well elucidated. Noticeably, the newly distinct porcine genogroup H RVs, recently identified in Japan, was found to cause diarrhea in pigs, as well as infecting humans in Japan, China, and Bangladesh60,61.
Molecular analyses of RV strains from neonates and young children with acute diarrhea in the metropolitan area of Belém, Brazil, have shown that such isolates contain NSP4 and VP4 genes, which are similar to RV strains of swine origin, suggesting a possible transmission between these species may have occurred37,56,62,63.
RVs are often isolated from diarrheic children and in general, transmission occurs via person-to-person transmission and through fomites. Nevertheless, several studies have shown that additional sources of transmission may include wastewater, causing recurrent infections, as well as contaminated food, especially in emerging countries21,64,65,66. In addition, the occurrence of symptomatic RV infections in young animals of many species of mammals has largely been reported, as well as in birds; noticeably, RVs can cause symptomatic and asymptomatic infections in cats67,68. Most RV infections in animals are reported to be subclinical, which suggests that their immune system may hamper the disease evolution toward a symptomatic stage69. However, it is known that orphaned animals under varying stress conditions have a greater immunological susceptibility, which favors the development of infections and the ensuing complications, which include co-infections involving other pathogens70. Nevertheless, the broad and still worldwide expanding spectrum of RV genotypes has evolved substantially throughout the past few decades; it may rather represent a challenge to both humans and animals, even enhancing the likelihood of interspecies transmission of unusual clinical presentations71.
RVs may be the primary cause of illness, or they can be associated with other pathogens. Of relevance in this context, the broad genetic diversity of RVs seems to be more evident in developing settings, probably related to several factors which include low hygiene levels and poor sanitation, depressed immune defenses, concurrent parasitic and enterovirus infections, and malnutrition among others. Additionally, the close contact between man, domestic animals (canines and felines), and domesticated animals (pigs and birds) may also account for such high prevalence of co-infections involving RV and other viral, bacterial, or parasitic pathogens21,36,72,73.
The pathophysiology of gastroenteritis associated with RV is multifactorial, involving distinct mechanisms namely malabsorption secondary to enterocyte damage and death, activation of the enteric nervous system, and villus ischemia74. All these factors contribute to the pathogenesis of diarrhea74,75. Furthermore, following a short incubation period, secretory diarrhea can result in induced by the enterotoxigenic potential of RV NSP4 non-structural protein, designated as the first viral enterotoxin21,75,76.
In this study, the use of Nitazoxanide for treatment of the diarrheic cats may have resulted in partial recovery of L. tigrinus with the passage of more pasty stools during the 13 days of treatment77,78,79. Although controversial remains as to the use of Nitazoxanide for viral gastroenteritis, there have been some reports supporting its use in RV and norovirus gastroenteritis in humans. Despite treatment, the disease further coursed with aggravation of physical depletion and soon progressed to death, possibly related to high serum levels of ammonia and other toxins associated with the aggravation that led to a fatal outcome74,75,76.
The epidemiological investigation of the present case is limited because, in parallel with the diagnosis of RV infection in quarantined orphaned cats, it was not possible to perform specific tests that could determine the true source of transmission. It remains to be determined, for example, whether transmission may have originated from a local, infected animal species that could act as asymptomatic carriers or if the transmission would have occurred through contact with contaminated water, food, or fomites67,80.
In view of the suspicion of an outbreak of human RVs in several states in Brazil and, despite the confirmation of the diagnosis of group A RVs gastroenteritis in the samples of L. tigrinus, it was not possible to undertake an epidemiological investigation that would possibly elucidate the origin of infection. However, it has been reported that the quarantine animal care-keeper of SV-PAMG was the owner of a domestic cat known to be hospitalized in a veterinary clinic in Belém a month before the onset of the symptoms of the wild cats, but it was not tested for RV. According to reports by small animal veterinarians in Belém, a significant number of feline patients suffering from clinical symptoms (diarrhea, vomits, and dehydration) compatible with those of RV disease were seen in the period when the two wild cats developed symptoms. These domestic cats were not tested for RV and had recovered after treatment for non-specific gastroenteritis, including antibiotic therapy (Enrofloxacin®), and fluid replacement (electrolytes and vitamin complexes). Although an unusual high prevalence of symptomatic human cases with diarrhea was reported by local health units during that time, additional evidence for zoonotic transmission is required to provide a more comprehensive understanding of the biological cycle of RV between humans and these animal species35,81.
In the present study, the samples were positive by ICG and ELISA tests, but negative by PAGE and RT-PCR for VP4 and VP7 genes using conventional set primers. PAGE showed the low sensitivity of this technique, corroborating the data of Guerreiro et al.56, Barros et al.37, and Duarte Jr et al.35 that also did not characterize the electrophoretic profile of RV in the positive samples (suggesting that if primers do not match the RNA of viral isolates, yields would be lower or even negative). Of note, the real-time PCR (qPCR) targeting the NSP3 gene has been shown to be more accurate, sensitive, and specific as compared to the conventional PCR assays, therefore greatly enhancing the detection of RVA strains in a variety of animal species82. By studying samples from animals located in areas with environmental degradation in Pará State, Brazilian Amazon, Barros et al.37 detected RVA by qRT-PCR with low viral load in different species, being 36% in domestic animals and 64% in wild animals. A subgroup of RVA-positive samples was subjected to RT-PCR for the VP4 gene to genotyping low viral loads samples according to Mijatovic-Rustempasic et al.83, resulting in the identification of P[6] and P[4] genotypes. More recently, Duarte Jr et al.35, analyzing fecal specimens of wild and exotic birds from the Amazon forest with different clinical signs hospitalized at the Veterinary Hospital of Universidade Federal do Pará, tested 23 samples by PAGE and RT-qPCR considering positive samples those with cycle threshold (CT) < 4084. The positive samples by this technique were amplified by RT-PCR/Nested reaction with low viral load for RVA (NSP3, VP7, VP4, and NSP4 genes), RVD (VP6 gene), RVF (VP6 gene), and RVG (VP6 gene) showing T2 genotype (NSP3 gene), G1, G3 equine like and G6 genotypes (VP7 gene), and P[2] genotype (VP4 gene), besides of co-infection for the RVA, RVD, and RVF groups. In addition, there are few reports of RV in domestic cats such as in the United Kingdom with RV detection in 3% of 1,727 cats surveyed38. Likewise, reports of RV infections in wild felids are also scarce, especially using next generation sequencing. In this sense, Johansson et al.85 investigated the intestinal virome of snow leopards in the Tost Mountains of Mongolia and detected RVA in 67% of the animals. The genotypes of RVA NSP1 and VP3 genes were determined as A3 and M2, genotypes that were also found infecting domestic cats.
In the present study, although the samples were recovered from animals with diarrhea, it was not possible the amplification of the VP4 and VP7 genes, and, consequently, the determination of RVA genotypes. Of note, this study has a limitation due to testing the samples using conventional primers. Thus, more sensitive assays are important to be implemented in research laboratories because there is a lack of tests for RVA diagnosis in animals since the current methods of RVA detection do not always detect RVA in these populations10. Furthermore, it was not possible to test the samples for other viruses (e.g. feline enteric coronavirus - FECV) or other diarrheagenic pathogens (e.g. with an emphasis on noroviruses).
It is worth mentioning that this study is the first in Brazilian Amazon Region which reports a confirmed case of group A RV infection in felines of the species L. tigrinus and L. pardalis. These findings should be regarded as preliminary and warrant further analyses of RV strains isolated from the study wild cats. It is intended that such further studies will comprise molecular analysis that would allow the identification of RV genotypes and subgroups involved in the transmission of the virus to the wild cats using more sensitive techniques such as qRT-PCR with low viral load followed by RT-PCR/Nested reaction with low viral load for RVA to the determination of several genotypes and NGS. It is worth expanding the search for RV strains infecting domestic and wild felines in the Amazon Region, due to the postulated potential of interspecies transmission involving humans. Overall, these proposed studies would offer prospects for a better understanding of the epidemiology of RV infection in this region.
CONCLUSION
The RV-positive results in two immunoassays support RVs as the aetiological agent of gastroenteritis of felids, leading to a rapid disease progression that coursed with non-specific symptoms and led to death. The histopathological lesions found in the small intestine of wild felids (hyperemic/swollen mucosa and so on) support the hypothesis of RV as being the etiological agent of acute gastroenteritis. These histopathological lesions are typical of RV infections according to previous studies in several animal models. This work complements other reports of RV in animals and warrants the conduct of further eco-epidemiological surveys in Amazon Region, mainly in felines, to better understand the chain of transmission of RV infections among susceptible hosts and to better determine the origin and evolution of these agents. Additional research studies should be planned so that continuous monitoring of animal species be made for the detection and molecular characterization of potential new circulating RV strains among humans, domestic and wild animals in the Amazon Region.














